Is Your MTT Assay Really the Best Choice?
Terry Riss
Promega Corporation
Abstract
The MTT assay was the first widely accepted method that replaced the radioactive tritiated thymidine incorporation assay to measure cell proliferation. However, there are several limitations associated with using the MTT assay. A better understanding of these limitations has influenced experienced assay development scientists to choose assay technologies that are better suited for their applications and have properties superior to MTT assays. This article briefly describes some of the limitations and disadvantages of the MTT assay method as well as highlighting alternative methods.
Introduction
Use of the MTT tetrazolium compound to measure the number of viable cells in culture was first described by Mosmann in 1983 (1) . The state of the art for high throughput screening (HTS) in the 1980s was transitioning into 96-well plates and the MTT assay represented the first homogeneous assay method that was useful for HTS. The broad adoption of this method was based on the simplicity of the homogeneous protocol, which includes adding two reagents to the assay wells, but does not require extra steps such as removing liquid or washing the cells that were necessary for radioisotope incorporation assays. For these reasons, the MTT assay was the first widely accepted method that replaced the radioactive tritiated thymidine incorporation assay used to measure cell proliferation.
Although the MTT assay has become widely used, it is often misused because of a lack of understanding of how the assay works and its limitations.
There are several limitations associated with using the MTT assay. A better understanding of these limitations has influenced experienced assay development scientists to choose assay technologies that are better suited for their applications and have properties superior to MTT assays. The following is a brief description of some of the disadvantages of the MTT assay method and complimentary or alternative methods that address these disadvantages.
How the MTT Assay Works
The MTT compound (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is soluble in culture medium and cell permeable. The reagent powder is typically prepared in phosphate buffered saline, added directly to cells in culture at a final concentration of 0.2–0.5mg/ml and incubated for 1 to 4 hours. Viable cells reduce the tetrazolium into a product which is a purple formazan precipitate that accumulates inside the cells and in the culture medium. A second reagent addition step solubilizes the formazan crystalline precipitate prior to quantitation by recording absorbance at 570nm. The amount of absorbance correlates with viable cell number.
MTT assays are rarely the best choice for estimating the number of viable cells in vitro.
Two-Step Procedure
Although the MTT assay is homogeneous, it requires two reagent additions and multiple steps. After the MTT reagent is added, the assay plates must be returned to the 37°C incubator for 1–4 hours for the signal to develop. Then the solubilization solution is added and absorbance is recorded. The development of alternative assays, such as the ATP assay (2–4), which involves fewer steps and does not require returning plates to the incubator has put the MTT assay at a disadvantage. The ATP assay’s simple add-mix-measure protocol has led to an increased adoption for high-throughput applications. The availability of assays that require fewer steps and eliminate returning plates to the incubator is probably the largest factor leading to decreased popularity of the MTT assay and increased adoption of the ATP assay (2–4).
MTS-based viability assays provide a homogeneous, add-mix-measure alternative for colorimetric viability assays.
Limitations of the MTT Assay
The original published method of the MTT assay describes the use of acidified isopropanol to solubilize the formazan crystals. The pH of the solubilization solution is adjusted to be acidic, which results in conversion of phenol red in the culture medium to the yellow form that avoids interference with recording absorbance of the formazan product. Although this formulation is still widely used, organic solvents may precipitate proteins from some serum-supplemented culture medium, and this may cause light scattering (5). Evaporation of volatile solvents and instability of the formazan signal are among the disadvantages related to solubilization solution formulations. The color of the acid-isopropanol solubilized formazan has been reported to be “stable for a few hours at room temperature” (1).
The literature describes several improvements to solubilization solutions including the use of DMSO, dimethylformamide, SDS, and combinations of detergents and organic solvents (1, 6–9). Although various organic solvents will slightly change the absorbance maximum of the formazan product, that shift is generally not a problem because of the broad nature of the absorbance spectrum (6). A combination of detergent and organic solvent adjusted to an acidic pH provides adequate solubilization and stabilizes the absorbance values for recording data several days later if necessary. However, it should be considered that including detergents in the solubilization solution may lead to increased problems with bubble formation in wells that result in variation in data among replicate samples. Appropriate disposal of microplates containing organic solvent can be an additional moderate disadvantage of the MTT assay. Each state and local institution likely has its own rules for organic solvent disposal that may need to be considered along with any special procedures for disposal of biological materials.
Lack of Sensitivity
The absorbance method of detection used by the MTT assay and other tetrazolium reduction assays (i.e., MTS, XTT, WST-1, WST-8) is generally less sensitive than fluorescent and luminescent methods for detecting viable cell number. This lack of sensitivity severely limits the ability to miniaturize the assay for HTS applications. Although the detection sensitivity varies widely among cell types and depends on the metabolic activity of the cell type being tested, typically tetrazolium reduction assays can detect 200–1,000 cells per well under optimal conditions. The sensitivity may be improved by optimizing the concentration of MTT and the incubation time with the cells; however, the incubation time is limited by the toxicity of the MTT reagent (10). In contrast, use of the luminescent ATP or the RealTime-Glo™ MT Cell Viability Assay to detect reducing capacity of viable cells results in detection of fewer cells per well and is more amenable to HTS applications.
MTT assays are frequently used to assess 3D microtissue cultures using Matrigel® substrate. However, due to its enhanced lytic capacity, the CellTiter-Glo® 3D Assay is better able to penetrate 3D microtissues.
Chemical Interference
Assay conditions that affect the chemical or enzymatic reduction of MTT result in increased background absorbance values and assay artifacts. A variety of chemical compounds are known to interfere with the MTT assay. These are mostly reducing compounds that lead to non-enzymatic reduction of the MTT to formazan. Examples include ascorbic acid, vitamin A, sulfhydryl-containing compounds including reduced glutathione, coenzyme A, and dithiothreitol (11–14). Chemicals that uncouple electron transport from oxidative phosphorylation of ATP also are known to interfere with the MTT assay (15). Plant extracts and polyphenolic compounds also have been reported to interfere with the MTT assay (16–17). Long-term exposure of MTT assay reagent to light and elevated pH of culture medium also may result in production of formazan and higher background absorbance. Appropriate controls (e.g., MTT assay reagent and test compound in culture medium without cells present) are necessary to detect assay chemistry interference. Alternatively, applying an orthogonal cell viability assay that detects a different marker can be used to confirm results. For example, detection of cytoplasmic aminopeptidase activity using the GF-AFC fluorogenic substrate (CellTiter-Fluor™ Assay (18)) could be multiplexed with the colorimetric MTT assay.
Toxicity
Although it is widely used, the MTT reagent exhibits cytotoxic effects, and adding the reagent to estimate cell viability may actually be damaging or even killing cells during the course of an experiment. MTT has been reported to be toxic to eukaryotic cells (10). Exposure of cells to MTT resulted in dramatic changes in morphology during the formation of formazan crystals (Figure 1). The MTT assay uses reducing equivalents such as the co-enzyme NADH to convert MTT into a colored formazan product. Diverting NADH away from critical cellular functions and toward reduction of MTT is likely to have adverse effects on cell health. Lü et al., speculated that the formazan crystals themselves may cause damage to membranes during exocytosis (19). Alternative assays measure different markers that are less critical for cell survival. The cell-permeable GF-AFC aminopeptidase substrate in the CellTiter-Fluor™ Assay is known to be a less toxic alternative, and the fluorescent signal that it produces provides more options for multiplexing with other assays (10, 18).
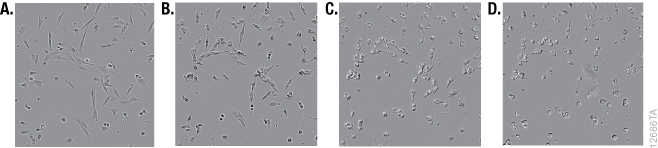
Tetrazolium Reduction Reflects Cell Metabolism and Not Cell Number
Although the MTT assay results generally correlate with the number of viable cells growing in standard culture conditions, the rate of tetrazolium reduction reflects the general metabolic activity or the rate of glycolytic NADH production (20–21). The rate of MTT reduction can change with culture conditions (e.g., pH and glucose content of medium) and the physiological state of the cells. For example, Con A-activated lymphocytes produce approximately 10 times as much formazan per cell as their normal counterparts (1). In addition, cells growing rapidly as a monolayer will have a different rate of metabolism than those that have undergone differentiation, grown into a confluent monolayer or have become senescent. The biological basis of the measured marker needs to be considered for all assays. An understanding of what makes the assays work (i.e., what cellular function or presence of a particular marker that contributes to generating a measurable signal) may help explain obvious artifacts.
Multiplexing Limitations
Multiplexing assays allows more information to be gathered from a single well. With the MTT assay, the addition of solvent to solubilize the formazan crystals destroys cell architecture and probably most enzymatic activity, limiting the ability to multiplex MTT with another assay, unless the other assay protocol precedes the addition of the solubilization solution. Using assays that do not kill the cells or that aren't based on accumulating signals, offers more options for multiplexing after cell viability is measured. Examples include the CellTiter-Fluor™ Assay, which uses the cell permeable GF-AFC protease substrate to measure cell viability (18), the RealTime-Glo™ Assay that measures reducing potential of viable cells in real time or the CellTox™ Green Assay, which is based on a non-permeant DNA binding dye that only stains dead cells (22).
Mitochondria Are Not the Sole Source of MTT Reduction
At the time that the MTT assay was developed, it was believed that the observed “properties are all consistent with cleavage of MTT only by active mitochondria” (1). This speculation of the direct involvement of succinate dehydrogenase was based on early work with inhibitors that act at selective sites in the mitochondrial electron transport chain (23). However, more recent research on the site of reduction of MTT has refuted the dogma that MTT is always reduced in the mitochondria (13, 20–21). Studies indicate that NADH is responsible for most MTT reduction and is associated not only with the mitochondria, but also the cytoplasm and associated with membranes in the endosome/lysosome compartment as well as the plasma membrane. MTT reduction at the plasma membrane may account for observations of formazan crystals occurring outside of cells.
Batch-to-Batch Variation of Reagents
The MTT powder is available from a variety of commercial vendors. Although the percent purity information is not always available, the quality of the MTT powder is generally adequate. However, a potential source of variability lies in the proper preparation, storage and handling of the MTT solution. Individual research labs may not have standard protocols for production and quality control testing of reagents that are seemingly easy to prepare. Proper labeling (including expiration dating), storage protected from light, and at the very least visual observation to confirm a clear yellow solution prior to use will help avoid experimental failures. Obtaining commercially prepared and QC-tested MTT reagent kits is an alternative to consider to avoid potential problems (6).
Alternative assay methods now exist for repeated real-time measurement of live cells and dead cells in the same sample by multiplexing different chemistries.
Alternatives to the MTT Assay
The most reliable and widely used alternative to the MTT assay is the ATP assay, which measures ATP as a marker of viable cells. The CellTiter-Glo® Luminescent Cell Viability Assay has the advantages of being the simplest, fastest, and most sensitive method for measuring viable cells using a plate reader. Whereas the MTT assay requires incubation of the tetrazolium substrate with viable cells for hours to generate a color signal (followed by a second procedural step to solubilize the formazan crystals), the ATP assay reagent immediately lysis cells upon addition and generates a stable luminescent signal following a 10 minute equilibration period. The ATP assay is by far the most sensitive method of measuring viable cells using a plate reader, with typical sensitivity that is two orders of magnitude better than the MTT Assay.
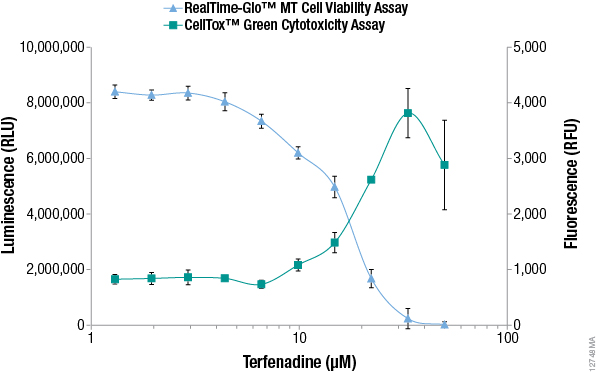
Additional alternative viability and cytotoxicity methods to MTT have recently been developed that enable monitoring of viable and dead cell numbers continuously in "real time". The RealTime-Glo™ MT Cell Viability Assay is performed by adding a reagent containing NanoLuc® luciferase and a pro-substrate directly to the culture medium (24). The pro-substrate cannot be used by luciferase until viable cells convert the pro-substrate into a luciferase substrate. The number of viable cells is proportional to the luminescent signal and typically can be recorded for up to 3 days after adding the reagent. The assay rapidly detects changes in viability. Figure 2 compares viability data measured with the RealTime-Glo™ MT Cell Viability to data from an MTT assay, demonstrating that the MTT assay data does not reflect what is happening in cells in real time.

Because the reagent does not kill the cells, a major advantage of this approach is the ability to multiplex with other assays or preserve samples for downstream analysis (detecting apoptosis, extracting RNA, detecting stress response markers, detecting dead cells, etc.) from the population of cells remaining in the sample well (25). For example, the number of dead cells can be measured in real time using the DNA binding dye CellTox™ Green, which is not toxic to viable cells. The dye is not permeable to viable cells, but stains "dead" cells that have lost membrane integrity. The dye can be added directly to cell cultures and fluorescence can be recorded repeatedly for up to 3 days to measure the accumulation of dead cells. See the CellTox™ Green Cytotoxicity Assay Technical Manual, #TM375, for details. Figure 3 shows multiplex measurement of live cells and dead cells from the samples highlighting the utility of new alternatives to the MTT assay.
Summary
Although historically the MTT assay has been widely used for a determining cell number, viability and health, a growing understanding of its many limitations is contributing to a shift to other cell viability assay methods. Alternative approaches with fewer protocol steps, greater detection sensitivity, the ability to record data repeatedly in real time and more effectively assay cells in 3D culture and the increased ability to multiplex with other assays all lead to a decrease in the use of MTT, once considered the standard method for measuring the number of viable cells in microplate assays.
Real-Time Cell Health Assays
ATP and MTT Assay Choices
The ATP based, luminescent CellTiter-Glo® Assay is the gold-standard for cell viability measurement. We also provide an improved MTS assay based on the MTT detection method, with a convenient one-solution format.
References
- Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63.
- CellTiter-Glo® Luminescent Cell Viability Assay Technical Bulletin, TB288, Promega Corporation.
- CellTiter-Glo® 2.0 Assay Technical Manual, TM403, Promega Corporation.
- CellTiter-Glo® 3D Cell Viability Assay Technical Manual, TM412, Promega Corporation.
- Twentyman, P.R. and Luscombe, M. (1987) A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 56, 279–85.
- CellTiter 96® Non-Radioactive Cell Proliferation Assay Technical Bulletin, TB112, Promega Corporation.
- Tada, H.et al. (1986) An improved colorimetric assay for interleukin 2. J. Immunol. Methods 93, 157–65.
- Hansen, M.B.et al. (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 119, 203–10.
- Denizot, F. and Lang, R. (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89, 271–7.
- Riss, T.L.et al. (2013) Cell Viability Assays. In: Sittampalam GS, Coussens NP, Nelson H, et al., editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences.
- Ulukaya, E., Colakogullari, M. and Wood, E. (2004) Chemotherapy 50, 43–50.
- Chakrabarti, R.et al. (2000) Vitamin A as an enzyme that catalyzes the reduction of MTT to formazan by vitamin C. J. Cell Biochem. 80, 133–8.
- Bernas, T. and Dobrucki, J. (2002) Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 47, 236–42.
- Pagliacci, M.et al. (1993) Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: a further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur. J. Cancer 29, 1573–7.
- Collier, A. and Pritsos, C. (2003) The mitochondrial uncoupler dicumarol disrupts the MTT assay. Biochem. Pharmacol. 66, 281–7.
- Bruggisser, R.et al. (2002) Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay.Planta Med. 68, 445–8.
- Han, M.et al. (2010) Limitations of the use of MTT assay for screening in drug discovery. J. Chinese Pharmaceutical Sci.19, 195–200.
- CellTiter-Fluor™ Cell Viability Assay Technical Bulletin, TB371, Promega Corporation.
- Lü, L et al. (2012) Exocytosis of MTT formazan could exacerbate cell injury. Toxicol. In Vitro 26, 636–44.
- Berridge, M.V.et al. (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 11, 127–52.
- Berridge, M.V. and Tan A.S. (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–82.
- CellTox™ Green Cytotoxicity Assay Technical Manual, TM375, Promega Corporation.
- Altman, F.P. (1976) Tetrazolium Salts and Formazans Prog. Histochem. Cytochem. 9, 1–56.
- RealTime-Glo™ MT Cell Viability Assay Technical Manual, TM431, Promega Corporation.
- Hook, B. and Bratz, M. (2014) RNA Isolation from 3D Microtissue Cultures: A Comparison of Manual and Automated Methods. Promega Corporation.
Acknowledgments
We wish to acknowledge the contributions of the following Promega scientists: Rich Moravec, Tracy Worzella, Brad Hook, Mark Bratz, Andrew Niles and Sarah Duellman.
Related Resources
A Viability Assay for 3D Cell Cultures
This article highlights a fast, simple assay designed for determining cell viability in 3D microtissue spheroids.
Multiplexing Cytotoxicity Assays with Viability Assays
Read this article to learn how to save time by multiplexing a real-time cytotoxicity assay with a viability assay.