Immunogenic Cell Death: Methods to Measure Damage Associated Molecular Patterns (DAMPS)
By Andrew Niles and Kevin Kupcho
Abstract
The study of cell death has taken many interesting turns over the last 50 years. It is now universally accepted that the mechanism by which cells die has major global consequences for the organism. As such, immunogenic cell death (ICD) has gained recent visibility for how it impacts the innate and adaptive immune responses. Damage associated molecular patterns (DAMPs) are specific biomolecular messengers produced during ICD, which mediate the progression and magnitude of this process. Therefore, proactive modulation of their presence has important ramifications in both health and disease.
Traditional assay methods for quantifying DAMPs provide reasonable informational value but can be laborious and time-consuming to implement. Here we describe two new bioluminescent assays (RealTime-Glo™ Extracellular ATP Assay and Lumit™ HMGB1 Immunoassay) which are fully homogeneous and can be employed on standard luminometry platforms. Together, these reliable and robust assays provide a convenient workflow for assessing experimental strategies to manipulate the in vitro immunogenic cell death response.
Introduction
Multicellular homeostasis is maintained in part by a collection of well-orchestrated, programmed cell death mechanisms. These programs are generally categorized into either “tolerogenic” or “immunogenic” modes. The tolerogenic form (also known as immunologically silent apoptosis) involves well-established intrinsic or extrinsic signaling cascades which lead to caspase activation and asymmetric lipid remodeling of the cell membrane. A crucial phosphatidylserine translocation event, from the inner to outer membrane surface, provides the necessary “eat me” signal whereby dying cells are phagocytized prior to loss of membrane integrity. This form of cell death does not promote inflammation and is generally associated with the efficient removal of aged, damaged, redundant, or unnecessary immune cells or other cell types during the developmental and tissue remodeling processes.
The inflammatory embodiment, known as immunogenic cell death, can be further segregated into subroutines such as necroptosis, pyroptosis, ferroptosis, NETosis, or a distinct form of apoptosis. Regardless of specific subroutine, DAMPs are exposed and/or released into the extracellular environment which perpetuate immune cell infiltration and subsequent localized inflammation. ICD is an important first line of defense for viral and bacterial attack and for the acquired development of tumor-specific cytotoxic T-cells. ICD can also be subverted to produce both acute and chronic pathologies.
Extracellular adenosine triphosphate (eATP) and high mobility group box B1 (HMGB1) protein are two DAMPs regarded as important for establishing the immunogenic cell death phenotype. Although principally known as a universal energy transporting molecule, the extracellular form of ATP is known to participate in a diverse array of cell to cell signaling events. eATP mediates these effects through P2 purinergic receptors expressed on a wide variety of cell types. In the case of ICD, eATP from the dying cell provides the initial chemoattractant gradient for dendritic cells (antigen-processing cells), then sustains the inflammatory event by paracrine release from responding immune cells.
HMGB1 is a major, chromatin-associated, non-histone protein which is compartmentalized in the nucleus. During an ICD event, HMGB1 translocates to the cytoplasm and eventually is released into the extracellular environment by either currently uncharacterized channel activation events or through passive release after a post-mortem loss of membrane integrity. Extracellular HMGB1 exists in various redox states that promote dendritic cell maturation and overall exacerbation of the inflammatory event.
Although each biomarker alone has predictive value for assessing the extent of an immunogenic cell death event, the combined absence or presence of eATP and HMGB1 provides a statistically stronger index for the phenotype than a single parameter measure. Therefore, the capability to rapidly and easily measure both biomarkers is key to discovery and development activities associated with the modulation of ICD.
Conventional Methods to Measure Immunogenic Cell Death
eATP and HMGB1 are released into the extracellular environment in a time-dependent manner during an ICD event. Currently, cell supernatant samples are periodically collected and processed over a time course by removing a volume of medium and subjecting it to low-speed centrifugation to remove cells and/or cell debris. The supernatants are then typically placed at +4 or -20°C until being interrogated for the level of specific analyte. Failure to reproducibly collect and process the supernatants, remove cells or debris, or store them appropriately, can complicate eATP and HMGB1 quantification.
Extracellular ATP is most commonly quantified by endpoint luminometry. By this method, a reaction mixture containing excess luciferase and luciferin is contacted with a sample containing unknown levels of eATP. The bioluminescent reaction produces photons of light proportional to eATP in the sample. However, accurate measurement of eATP from supernatants represents a particularly monumental challenge. For instance, most transformed cell lines have enhanced expression of cell membrane resident ATPases (e.g., CD39) and are propagated and maintained in serum which also contains significant ATPase activity. These ecto-pyrase activities put experimentally released eATP concentrations at particular risk during sampling and processing, by degrading eATP levels dramatically within a matter of minutes (Figure 1).
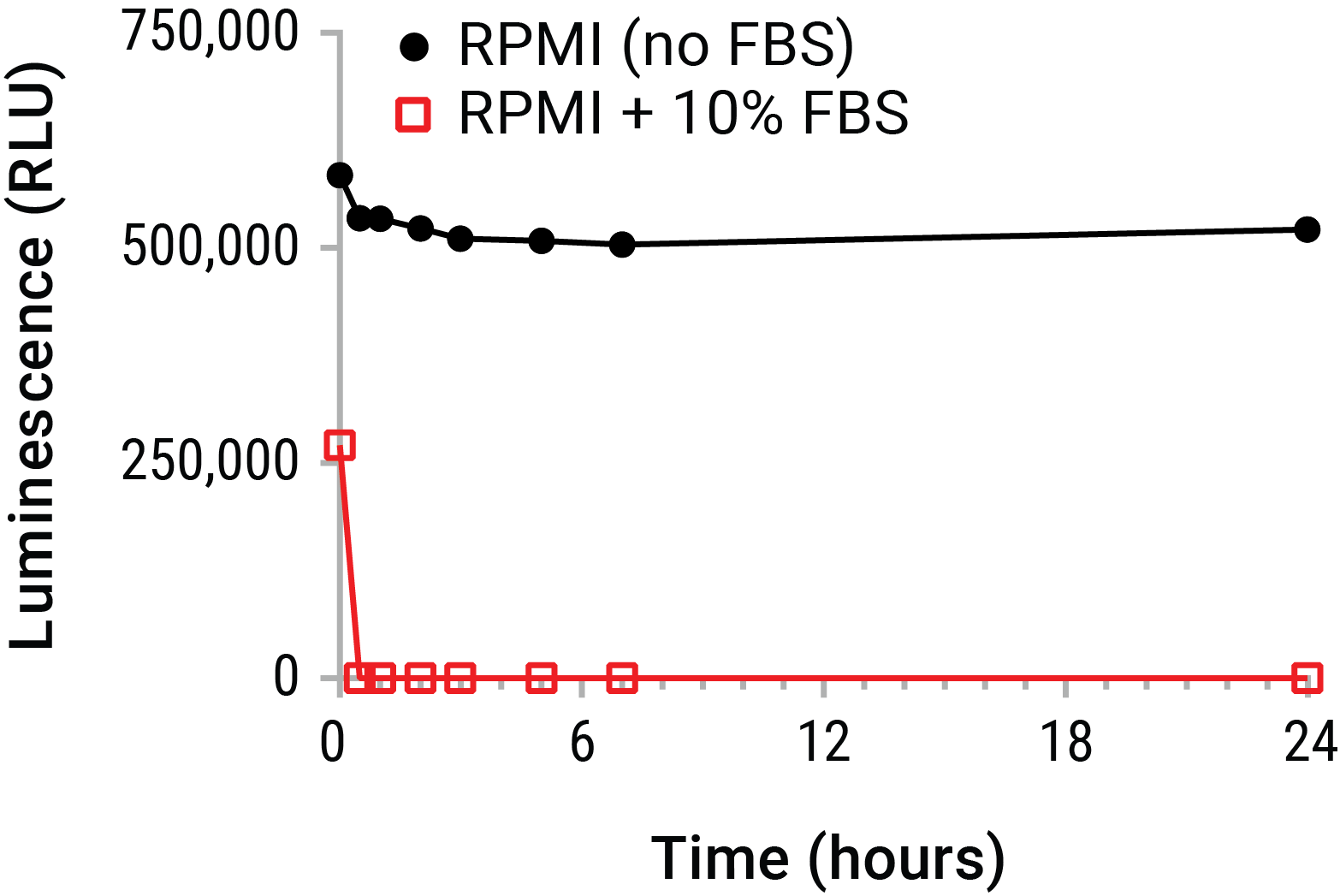
Figure 1. Extracellular ATP is unstable in cell culture environments. Purified ATP (20nM) was spiked into RPMI 1640 with or without 10% FBS and placed on ice. Samples were periodically removed and incubated at 37°C over a 24 hour time course. ATP was measured from all samples at the time course completion using the CellTiter-Glo® Luminescent Cell Viability Assay. The data emphasize that standard supernatant collection strategies risk substantial eATP decay in short time periods. This decay prior to assay may greatly underestimate eATP levels. A real-time measurement of eATP prior to decay is therefore indicated.
HMGB1 levels are typically measured by Western blot or ELISA. While Western analysis provides key biochemical proof of analyte release, it is poorly quantitative, tedious, and laborious. ELISAs are also tedious and time consuming but provide exceptional sensitivity. Unfortunately, ELISA formats also suffer from poor dynamic range. This limited dynamic range requires samples to be diluted prior to testing to fall within the assay’s linear range. When the analyte level of the sample is unknown, the sample must be run neat and at various dilution levels, increasing the number of samples to be processed. Further, at least one well-validated commercial vendor’s assay can be cost-prohibitive.
Improved Methods to Measure Extracellular ATP and HMGB1
We have greatly streamlined the ICD analysis workflow by developing two new, homogenous assay chemistries which do not require sample collection and processing. You can simply create the respective reagents and deliver them to parallel experimental sample culture wells. The RealTime-Glo™ Extracellular ATP Assay reagent contains thermal-stable Ultra-Glo® luciferase and chemically-pure luciferin which have been formulated to be compatible with live cells (Figure 2). These components are mixed with growth medium and added at time “zero” to treated and untreated cells to detect eATP release as a function of time (Figure 3). These reactants have been carefully optimized for real-time tolerability, so the inherent biology of untreated cells is unperturbed and a baseline of eATP release can be established. Aside from the considerable time and effort savings provided by the new format, the real-time readout provides a continuous and contemporary reporting of eATP levels throughout the exposure. When employed in an automated format using heated luminometers in kinetic mode, using either environmental gas control or CO2-independent medium, the assay becomes truly “hands-off” through the final exposure time point (Figure 4).

Figure 2. The bioluminescent extracellular ATP detection chemistry.

Figure 3. The RealTime-Glo™ Extracellular ATP Assay workflow.
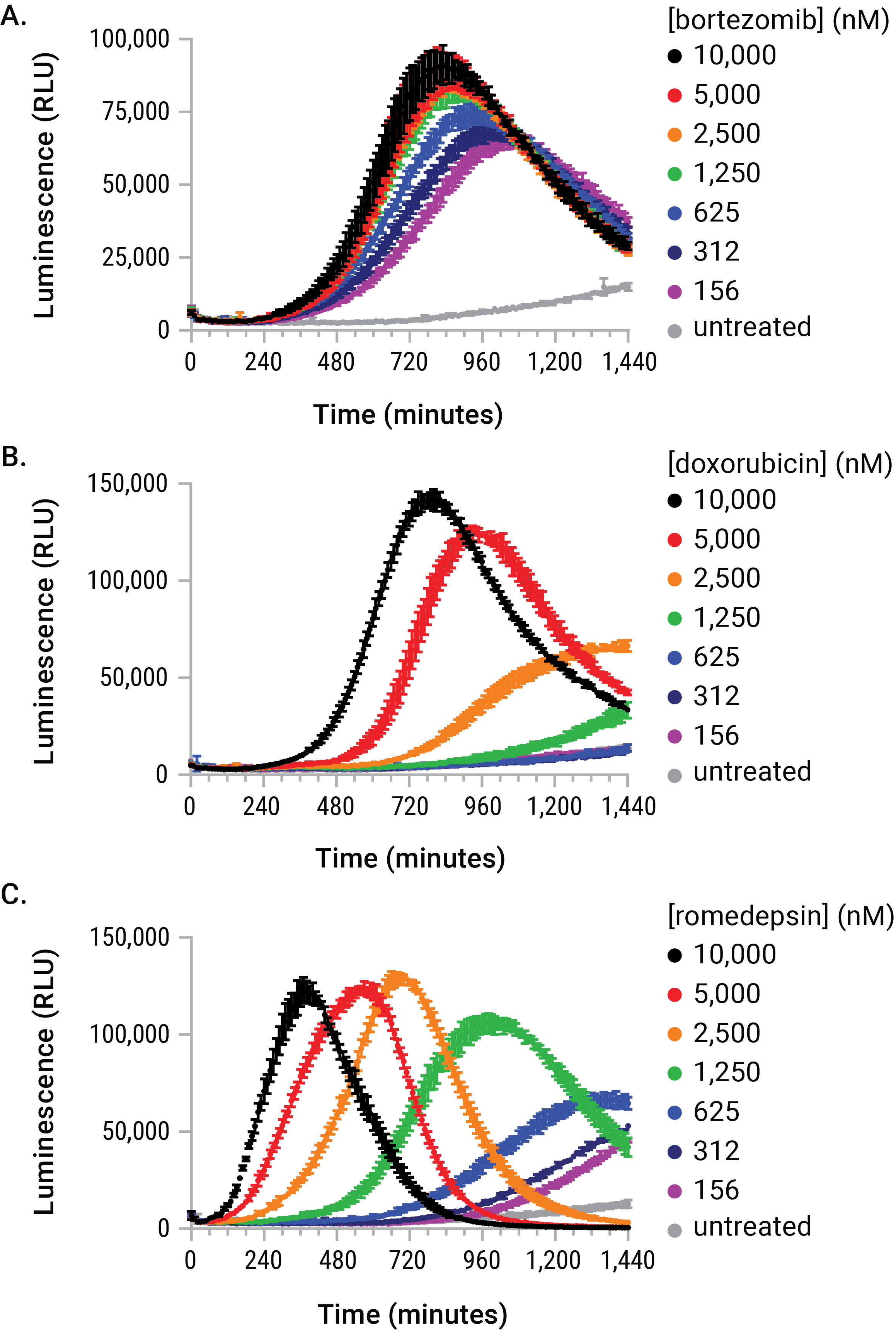
Figure 4. The stimulus-driven release of eATP is dose- and time-dependent. U937 cells were dosed with serial dilutions of bortezomib, doxorubicin, and romidepsin in the presence of the RealTime-Glo™ Extracellular ATP Assay reagent. The eATP release profiles generated during the exposure are kinetically unique with respect to dose, time, and signal magnitude. These differences would have made arbitrary endpoint eATP determinations difficult to interpret and likely inaccurate.
The Lumit™ HMGB1 Immunoassay reagents can be added directly to test wells at the exposure completion. The reagent contains a luciferase substrate and two anti-HMGB1 monoclonal antibodies which have been conjugated to complementary elements (LgBiT or SmBiT) of a rationally split and re-evolved luciferase (Figure 5). In the absence of HMGB1 analyte, the antibody conjugates remain free and unbound in solution and produce only modest luminescence signal. In the presence of HMGB1, the antibodies bind to their cognate epitopes and facilitate proximity-induced complementation of LgBiT and SmBiT on the antigen. The result of these events is luminescence proportional to the amount of analyte. A compound’s ICD-inducing properties can therefore be described by following potency and magnitude of response versus time (Figure 6). Notably, HMGB1 antigen detection after ICD-induced release events can be measured using either unprocessed cell samples or supernatants using the Lumit™ method. Both sample types correlate well with existing ELISA methods (Figure 7). Further, the induced and uninduced values derived from the experiment can be compared to a standard curve of recombinant human HMGB1 provided in the kit. Additional experimentation has demonstrated significant cross-reactivity with the mouse isoform of HMGB1, allowing for its analysis in similar induction systems.
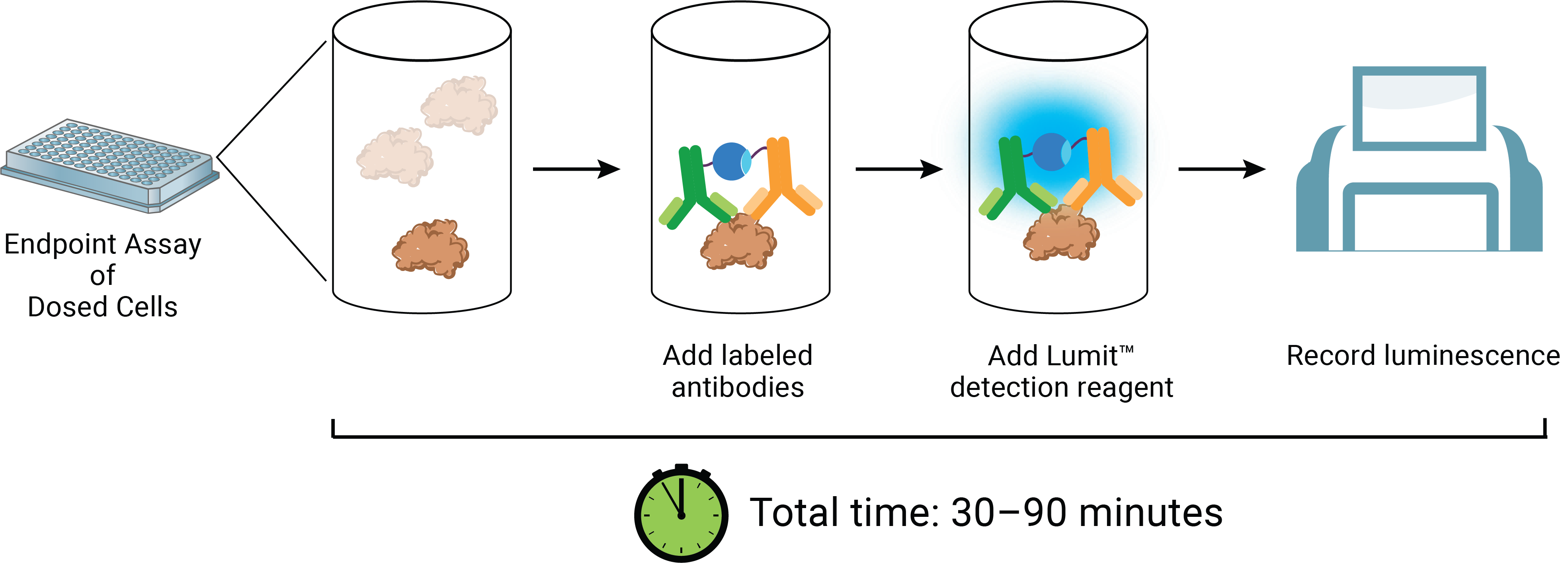
Figure 5. The Lumit™ HMGB1 Immunoassay concept and workflow.
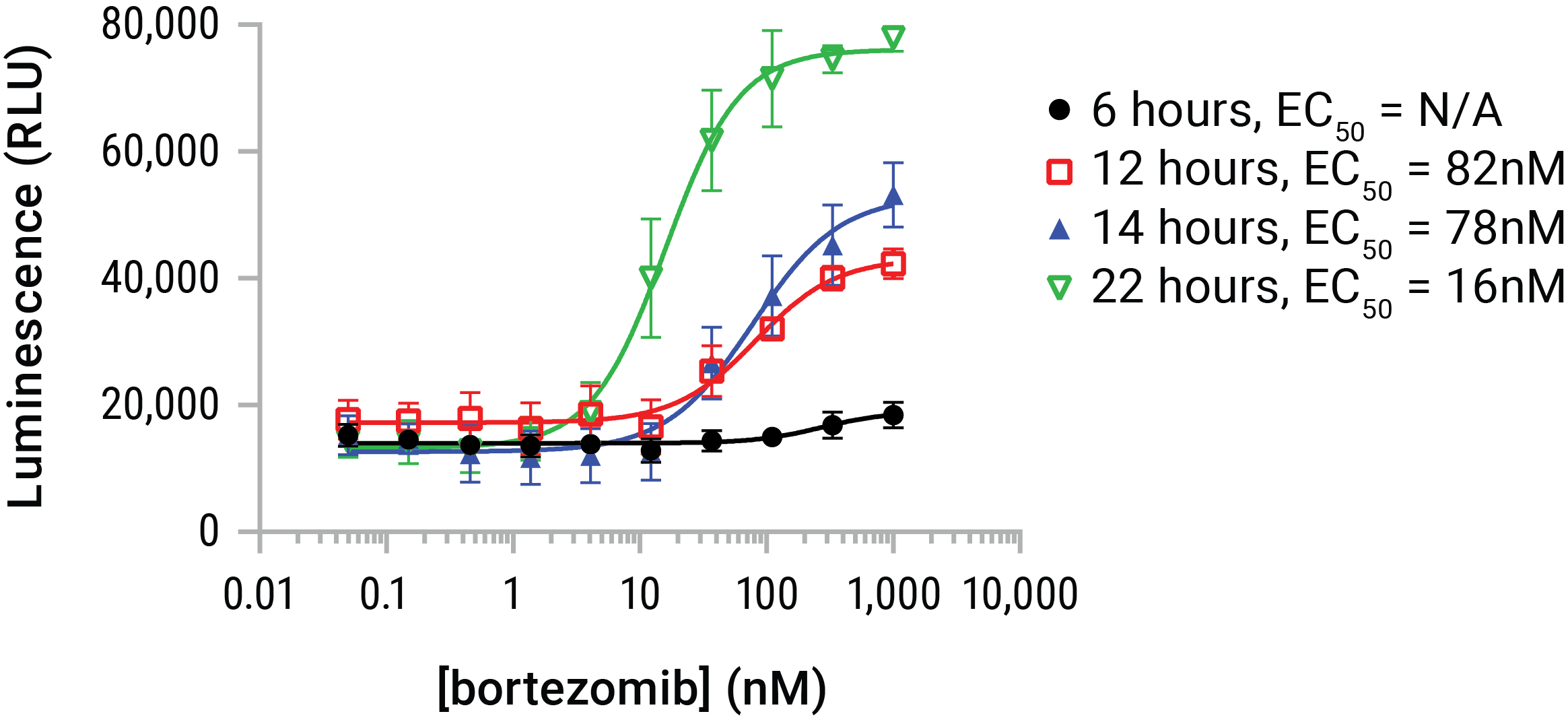
Figure 6. HMGB1 release is dose- and time-dependent. U937 cells were dosed with serial dilutions of bortezomib in a time course that represented 6, 12, 14, and 22 hours of compound exposure. The Lumit™ HMGB1 Immunoassay reagent was added at endpoint and luminescence data gathered. Bortezomib began initiating HMGB1 release by ~6 hours and continued to affect additional release throughout the 22-hour time course. HMGB1 release was additive with time in this model, but HMGB1 can be prone to loss of antigenicity in extended exposures. Therefore 24- and 48-hour exposures are typically indicated for compounds of unknown potential or potency.
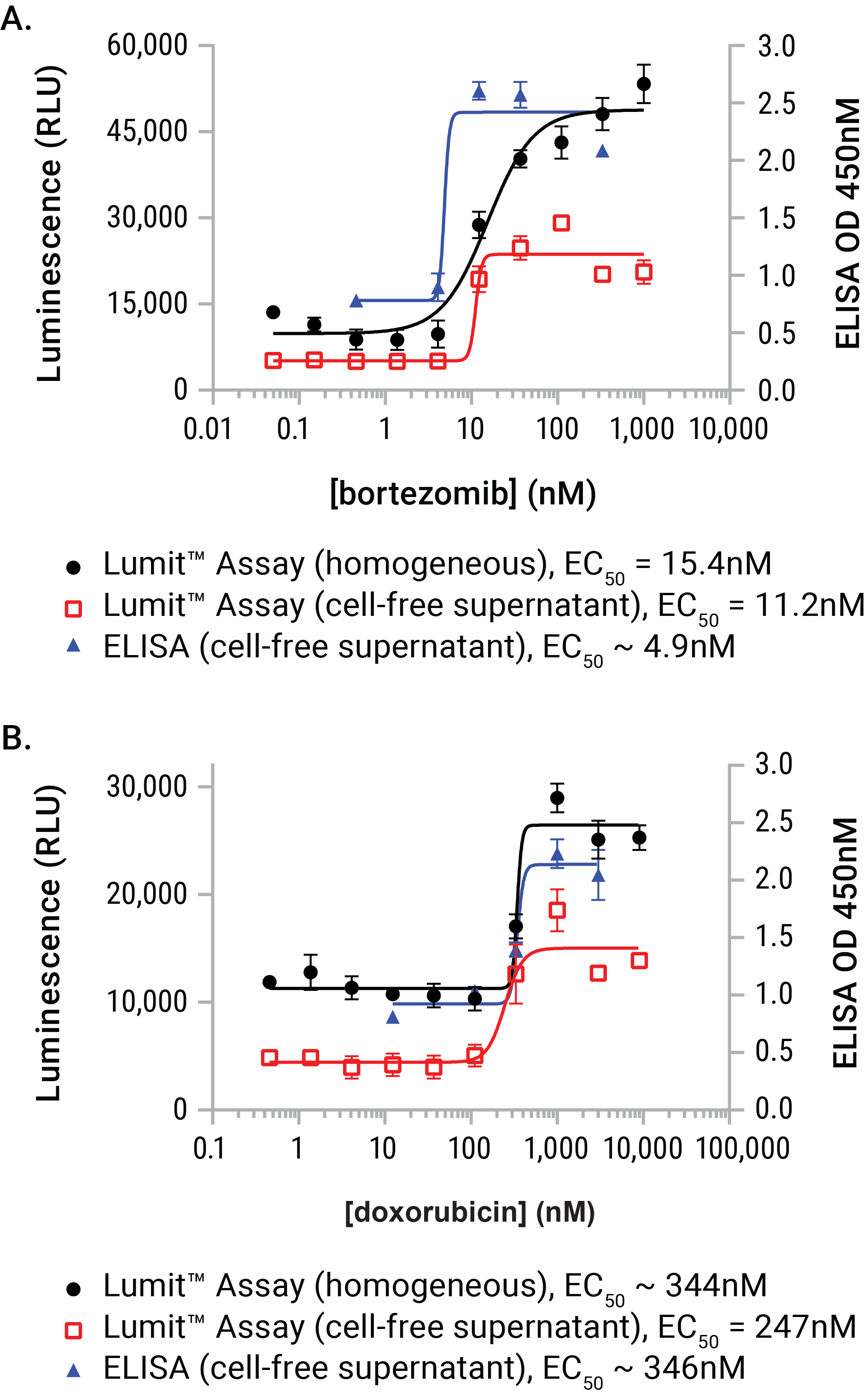
Figure 7. A performance comparison between the Lumit™ HMGB1 Immunoassay and a commercial ELISA vendor. U937 cell were dosed with either bortezomib or doxorubicin for a period of 24 hours. At exposure completion, cell-free supernatants were prepared from parallel wells of the plates. Lumit™ HMGB1 Immunoassay reagent was added to either wells containing treated cells or the collected supernatants. Supernatants (and dilutions of supernatants) were also assayed using a commercial HMGB1 ELISA. The two sample formats demonstrated exceptional concordance to the commercial ELISA method and required substantially less time to gather the results (1.5 hours with the Lumit™ HMGB1 Immunoassay as opposed to 25.5 hours with ELISA).
Method Validation Using ICD Tool Compounds and Model Cell Lines
The RealTime-Glo® Extracellular ATP Assay and Lumit™ HMGB1 Immunoassay were designed for use during in vitro ICD discovery and characterization efforts. Therefore, we validated the assays using a variety of human (Figure 8) and mouse (Figure 9) suspension and attachment-dependent cell lines dosed with serial dilutions of DAMP-inducing tool compounds. Data for the ATP release experiments were collected in real time using either a BMG CLARIOstar™ equipped with atmospheric control or with the CLARIOstar™ using CO2-independent medium. Real-time eATP data and Lumit™ HMGB1 data were collected using the GloMax® Discover.

Figure 8. Dual DAMP measurements in a human cell line. U937 cells were dosed with serial dilutions of mitoxantrone in parallel plates. RealTime-Glo™ Extracellular ATP Assay reagent was added to one plate at the time of dosing in CO2-independent medium with 10% FBS and signals collected over a 24-hour time period using the GloMax® Discover. The second plate was incubated for 24 hours, then contacted directly with the Lumit™ HMGB1 Immunoassay reagent. Luminescence was collected using the GloMax™ Discover. Top Panels: Mitoxantrone produced a dose- and time dependent release of eATP, which produced a progressive increase in potency over the time course. Bottom Panels: Recombinant human HMGB1 antigen produced a linear response from 1ng to 729ng/ml. Mitoxantrone elicited a dose-dependent HMGB1 release event with a potency in agreement with that of eATP.

Figure 9. Dual DAMP measurements in a mouse cell line. EL4 cells were dosed with serial dilutions of idarubicin in parallel plates. RealTime-Glo™ Extracellular ATP Assay reagent was added to one plate at the time of dosing in CO2-independent medium with 10% HS and signals collected over a 24-hour exposure using the GloMax® Discover. The second plate was incubated for 24 hours, then contacted directly with the Lumit™ HMGB1 Immunoassay reagent. Luminescence was collected using the GloMax™ Discover. Top Panels: Idarubicin produced a dose- and time dependent release of eATP, which produced only modest increases in potency over the time period. Bottom Panels: Recombinant mouse HMGB1 antigen produced a linear response from 3ng to 2,187ng/ml. Idarubicin elicited a dose-dependent HMGB1 release event substantially more potent than that of eATP.
Summary
The homogeneous, add-mix-measure format of the two new assays for immunogenic cell death dramatically increases throughput and reduces effort during discovery and characterization activities for new ICD-inducing compounds. This efficiency is achieved by avoiding unnecessary sample collection and processing steps, and by eliminating detection-related incubations and washes.

