Homogeneous Assays For Triglyceride Metabolism Research
Kimberly Haupt and Mike Valley
tpub_221, Dec 2020
Abstract
Measuring triglycerides and their metabolism is important for understanding metabolic disease mechanisms. Conventional methods for measuring lipid metabolism—such as staining, colorimetric or fluorometric assays, and mass spectrometry—are useful, but each method has its own limitations. Here, we introduce two new assays based on sensitive bioluminescent detection: Triglyceride-Glo™ Assay and Glycerol-Glo™ Assay. These assays do not require organic extraction for sample preparation and do not involve any wash steps, drastically simplifying your workflow while providing reliable, quantitative data.
Introduction
Mammals use lipids, chiefly triglycerides, for long-term energy storage. Triglycerides are synthesized to store excess energy after meals and are broken down during periods of fasting to provide energy to all the cells of the body. Triglyceride synthesis (lipogenesis) and breakdown (lipolysis) are tightly regulated by hormone-mediated signaling cascades.

Figure 1. Lipogenesis and lipolysis. A triglyceride molecule is composed of three energy-dense fatty acids esterified to one molecule of glycerol. Triglyceride synthesis is called lipogenesis, while triglyceride breakdown is called lipolysis.
The dysregulation of triglyceride metabolism is observed in many disease states including diabetes, obesity, liver diseases (e.g., liver steatosis, NAFLD, NASH) and cancer. To better understand and develop treatments for these diseases and others, researchers need tools to monitor triglyceride metabolism in biological samples. Lipogenesis is measured by detecting changes in triglyceride levels, while lipolysis is typically measured by detecting changes in glycerol levels.
Conventional Methods to Detect Triglyceride Metabolism
There are several common methods to detect triglycerides and measure their metabolism in biological samples:
Staining
Various inexpensive stains can be used to assess lipid levels in tissue or cell culture samples (e.g., Oil Red O, BODIPY® dye). Staining can be visualized by microscopy, and the progression of lipolysis or lipogenesis can be inferred. However, staining protocols are lengthy, with multiple wash steps, and are not amenable to high-throughput applications. In addition, it is not possible to distinguish between triglycerides and other neutral lipid species with this method.
Colorimetric and Fluorometric Plate-Based Assays
These microplate-based detection kits use a cocktail of enzymes to convert triglycerides or glycerol into a detectable colorimetric or fluorescent signal. These assays can be used to quantitate metabolism and are compatible with a wide variety of sample types. However, they have a limited linear range and may not be sensitive enough to detect physiologically relevant changes in lipid levels. In addition, sample preparation can require organic extraction, which uses toxic solvents under extreme temperature conditions and requires centrifugation.
Mass Spectrometry
Spectrometric methods can be used to give a complete picture of the “lipidome”—all the lipid species present in a biological sample. These experiments generate large quantitative data sets, which can be used to characterize lipid metabolism processes. However, mass spectrometry is expensive and requires specialized instrumentation and technical expertise. Such large, untargeted data sets are time-consuming to analyze and may go beyond the scope of your needs.
Bioluminescent Assays to Monitor Triglyceride Metabolism
We have developed new assays that offer an easier way to investigate and monitor triglyceride metabolism: Triglyceride-Glo™ Assay and Glycerol-Glo™ Assay. These add-and-read assays are compatible with a wide variety of sample types, including lysed cells, culture medium, tissue homogenates, serum, and lipoprotein fractions. Their luminescent readout is more sensitive than colorimetric or fluorometric plate-based assays and gives reliable and accurate quantitation of metabolic changes.
At the core of both assays is the correlation of glycerol molecules and light production. For the Triglyceride-Glo™ Assay only, an initial lipase treatment is required to convert triglycerides to glycerol. Then, in both assays, a pair of enzymes (glycerol kinase and glycerol-3-phosphate dehydrogenase) oxidize glycerol to generate NADH. Next, a reductase enzyme uses NADH to convert its proluciferin substrate into luciferin. Finally, Ultra-Glo™ rLuciferase oxidizes luciferin to produce visible light. The amount of light produced can be measured using a luminometer, and correlates to the quantity of triglycerides/glycerol in the starting sample. The Ultra-Glo™ rLuciferase enzyme has been engineered for bright, sustained light emission regardless of detergents, reducing agents, or temperature fluctuations, so the assay output is robust for any sample type.

Figure 2. Schematic diagram of Triglyceride-Glo™ and Glycerol-Glo™ Assays. Lipase step occurs only in the Triglyceride-Glo™ Assay.
The Triglyceride-Glo™ and Glycerol-Glo™ Assays have a simple protocol: just add reagents to your sample, incubate, and read the plate with any plate-reading luminometer (Figure 3). No organic extraction, extreme temperatures, or high-speed centrifugation are required to prepare samples. Instead, the assays use a simple 30-minute detergent lysis step. For cell culture experiments, detection reagents can be added directly into the wells containing cells, or they can be added to media samples in a separate plate. The latter approach leaves the cells available for additional downstream assays, such as measuring other metabolites or cell viability. In addition, Triglyceride-Glo™ and Glycerol-Glo™ Assays can be used in 384-well plates for high-throughput screening applications.

Figure 3. Simple, homogeneous workflow for Triglyceride-Glo™ and Glycerol-Glo™ Assays.
| Lipid Metabolism Detection Method | Advantages | Disadvantages |
|---|---|---|
| Staining |
|
|
| Colorimetric/Fluorometric Assays |
|
|
| Mass Spectrometry |
|
|
| Promega Bioluminescent Assays |
|
|
Results from our bioluminescent lipid metabolism assays can confirm and complement other methods, especially those with qualitative readout. Figure 4 shows how adipocyte differentiation and lipogenesis can be monitored in parallel using the Triglyceride-Glo™ Assay and Oil Red O staining.
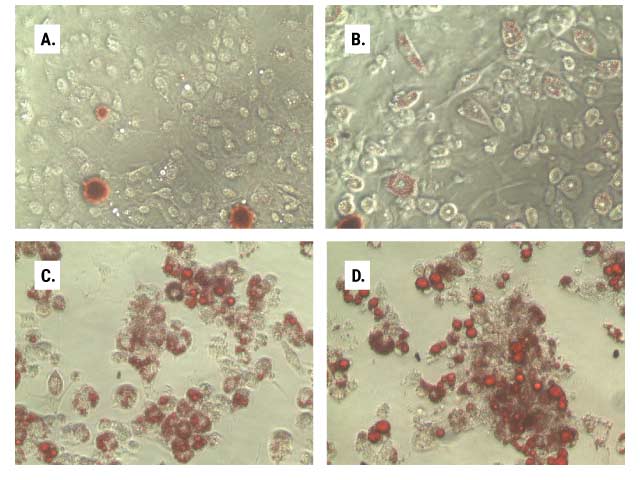

Figure 4. Lipid accumulation during adipocyte differentiation. In parallel 96-well plates, we monitored the lipid accumulation of 3T3L1-MBX cells as they were differentiated from fibroblasts (Panel A, day 5) through differentiation stages 1 (Panel B, day 12) and 2 (Panel C, day 14) to mature adipocytes (Panel D, day 21). One set of plates was stained with Oil Red O (Panels A–D), and the other was assayed according to the Triglyceride-Glo™ Assay protocol (Panel E). At each stage of differentiation, after media removal, cell washing and lipase treatment, 2.5µl aliquots of the treated samples were diluted into 47.5µl of Glycerol Lysis Solution and assayed per the standard protocol. Data are the average of six replicates, and triglyceride was determined by measuring total glycerol levels.
Monitoring Insulin Activity in Diabetes Research
Insulin is a critical regulator of lipid metabolism. In hepatocytes and adipocytes, it promotes lipogenesis and inhibits lipolysis. With Triglyceride-Glo™ and Glycerol-Glo™ Assays, you can monitor the effect of insulin and other effectors on lipid metabolism. In Figure 5, the Glycerol-Glo™ Assay was used to monitor lipolysis in adipocytes. Treatment with isoproterenol increases lipolysis above the basal level. Treatment with both isoproterenol and insulin also results in an increase, but the increase is less than isoproterenol alone. In this way, the Glycerol-Glo™ Assay can be used to assess both activators and inhibitors of lipolysis.

Figure 5. Insulin-mediated inhibition of glycerol release in adipocytes. Adipocytes differentiated from 3T3-L1 MBX cells were treated with combinations of isoproterenol (25nM) and insulin (150nM). After 90 minutes of treatment, a sample of the media was removed from each well and assayed using the Glycerol-Glo™ Assay. Each condition was tested in triplicate and the error bars represent standard deviation.
Monitoring Steatosis in Liver Disease Research
BSA-bound fatty acids and lipoproteins stimulate intracellular triglyceride accumulation in hepatocytes via lipogenesis. Here, we used the Triglyceride-Glo™ Assay to monitor triglyceride content of human liver 3D microtissues across several treatment conditions and over time. The results demonstrate that the Triglyceride-Glo™ Assay can be used to monitor the progression of steatosis in cell models.

Figure 6. Triglyceride levels in human liver microtissues. 3D InSight™ Human Liver Microtissues (InSphero) were incubated for 3 and 10 days in serum-free medium containing either physiological (LG/LI) or supraphysiological (LG/HI) levels of glucose and insulin and supplementation with either free fatty acids bound to BSA (FFA) or low density lipoprotein plasma fraction (LDL). The microtissues were washed twice in PBS and assayed for total glycerol content according to the Triglyceride-Glo™ protocol. Values were plotted as concentration of triglyceride per microtissue (MT). The data were generously provided by InSphero, AG, Zurich, Switzerland.
Summary
The Triglyceride-Glo™ and Glycerol-Glo™ Assays offer a better method for researchers who need to measure lipolysis and lipogenesis. Their simple, no-wash protocol results in more sensitive bioluminescent detection than colorimetric or fluorescent plate-based assays. Instead of cumbersome organic extraction, the assays use a quick detergent lysis step to prepare lipid samples. These bioluminescent assays can stand alone or complement other lipid detection methods.
Acknowledgements: We are grateful to our collaborators at InSphero for providing the human liver microtissue data shown in Figure 6.