Current Gold-Standards for MSI Testing and the Clinical Relevance of the MSI-H Biomarker
Continuing education credit is available for this activity. For more information on how to receive credit, please see details at the bottom of the page.
Lindsay Megenhardt, PhD., CHES
Medical Affairs
What Are Microsatellites?
Microsatellites are short, repetitive sequences of DNA consisting of mononucleotide, dinucleotide, or longer repeat units. These sequences are inherently prone to replication errors due to DNA polymerase slippage. Under normal circumstances, key DNA mismatch repair (MMR) proteins, including MLH1, MSH2, MSH6, and PMS2 correct these errors. However, when the MMR system is defective—whether due to genetic mutations or epigenetic silencing—these errors accumulate, leading to microsatellite instability (MSI). See a video of how the MMR system works
One of the most common causes of MSI in sporadic cancers is hypermethylation of the MLH1 promoter, leading to gene silencing. In contrast, germline mutations in MMR genes underlie Lynch syndrome, a hereditary condition predisposing individuals to multiple malignancies.
Detecting Microsatellite Instability
Historical Techniques
Initially, MSI detection involved polyacrylamide gel electrophoresis following polymerase chain reaction (PCR) amplification of tumor and normal DNA. Differences in banding patterns between these samples indicated MSI. While effective, this method has largely been replaced by more sophisticated approaches.
PCR and Capillary Electrophoresis: The Gold Standard
The current gold standard for MSI detection involves PCR amplification of microsatellite loci, followed by capillary electrophoresis (Yamamoto & Imai 2019). Fluorescently labeled primers amplify specific loci, and peak shifts between tumor and matched normal samples indicate MSI. A panel including five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) has been shown to increase sensitivity and specificity (Mao et al. 2021).
Immunohistochemistry (IHC) for MMR Proteins
Immunohistochemistry (IHC) analysis of MMR protein expression serves as an alternative MSI detection method. This technique identifies the presence or absence of the four major MMR proteins (MLH1, MSH2, MSH6, or PMS2) in tumor tissue. While IHC provides rapid and cost-effective results, it may yield false negatives due to biological factors, such as nonfunctional proteins that remain detectable upon staining (Bacher et al. 2016; Dudley et al. 2016; Evrard et al. 2019; McCarthy et al. 2019).
Next-Generation Sequencing (NGS)
Next-generation Sequencing (NGS) enables comprehensive genomic profiling, including MSI detection. By analyzing mutation burden and microsatellite alterations across the genome, NGS offers a broader view of genomic instability. However, standardization and validation remain challenges for routine clinical application (Gilson et al. 2021; Umar et al. 2004).
Single Biomarker Testing vs. NGS

Comparing NGS and PCR Workflows: Turnaround Time & Applications
NGS and PCR workflows differ significantly in turnaround time and can be influenced by lab equipment, workflow, and sample volume. NGS begins with DNA extraction and quality assessment, followed by diverse gene enrichment methods affecting sequencing setup. Sequencing depends on chemistry, platform, and lab throughput, often requiring batch processing. Bioinformatics analysis ensures quality control, aligning reads and assessing MSI status (MSI-high, MSI-stable, or MSI-indeterminate). NGS is typically part of broader tumor profiling and provides extensive variant data, informing treatment strategies and clinical trial eligibility.
In contrast, PCR follows a streamlined four-step process: DNA isolation, quality checks, PCR amplification, capillary electrophoresis, and automated peak analysis. With a targeted approach, PCR delivers results in 1–2 days, making it efficient for single-biomarker detection.
MSI workflow comparison


For advantages and limitations of these detection methods visit our Methods of Comparison Table
Defining MSI
According to the National Cancer Institute (NCI), MSI-high (MSI-H) status is determined by instability in at least two out of five loci while deficient mismatch repair (dMMR) is identified by the absence of one or more MMR proteins in tumor tissue. The tables below highlight the standard classification for MMR and MSI. It is important to note that many labs have forgone the use of the MSI-Low (MSI-L) classification due to no observed clinical differences seen between MSI-L and MSI-Stable (MSS) result and therefore a binary classification of MSI-high or MSI-stable is most commonly reported (Umar et al. 2004).
Classification for MMR by IHC and MSI by PCR
Mismatch Repair Deficiency (dMMR)
| MMR Result | Status | Tumor Findings |
|---|---|---|
|
MMR deficient |
dMMR | 1 or more MMR proteins are absent (not expressed) based on IHC and lack of tumor tissue staining. Sample is considered defiecient for MMR. |
|
MMR proficient |
pMMR | All MMR proteins are expressed based on IHC and sample is then considered proficient for MMR |
Classification for MMR by IHC and MSI by PCR
Microsatellite Instability (MSI)
| MMR Result | Status | Tumor Findings |
|---|---|---|
|
MMR high |
MSI-H | Shift in ≥2 of five tumor loci when compaed to non-neoplastic tissue or when ≥30% of loci within a PCR panel demonstrate instability |
|
MMR low |
MSI-L | <30% or 1 of the loci are unstable* |
|
MMR stable |
MSS | No loci are unstable |
For MSI testing, sometimes when a larger panel is used, a shift of ≥30% of loci is required for MSI-H, a shift in one locus (≤30% of loci in larger panels) is classified as MSI-L, and when no instability detected (≤10% loci in larger panels), the tumor is considered MSS. Not all labs report or define MSI-L, and some include MSI-L with MSS. No observed difference in clinical and histological parameters such as percentage of mucin, histological type or grade has been observed between MSI-L vs MSS samples.
Characteristics of MSI-High Tumors
MSI-H colorectal tumors exhibit distinctive pathological and immunological features (Mei et al. 2022):
- Typically demonstrate loss of MMR protein function
- Upregulated expression of inhibitory immune checkpoint proteins
- Prominent immune infiltration of Tumor-Infiltrating Lymphocytes (TILs)
- Distinct histology with poor differentiation with pushing margins, mucinous, lack of dirty necrosis, diploid in tendency with preserved chromosomal architecture
Prevalence Across Cancers
MSI-H has been identified across a spectrum of solid tumors, yet its prevalence varies. For instance, in colorectal, gastric, and certain gynecological cancers, such as endometrial, uterine, and ovarian cancers, MSI-high is detected in over 10% of cases (Lorenzi et al. 2020). MSI-H is also observed in other solid tumors, albeit less frequently. This includes various sarcomas, gliomas, as well as pancreatic, prostate, thyroid, and breast cancers, among others. However, in certain cancer types, like lung cancer, the prevalence of MSI-H is not as well established and continues to be an active area of research.
MSI Prevalence Across Solid Tumors

MSI-H has been identified in colorectal, head and neck squamous cell carcinoma, non-small cell lung cancer, squamous and basal cell skin cancer, melanoma, gastric, bladder, endometrial and ovarian cancer, hepato-pancreobiliary, and other solid tumours. Higher incidence in endometrial, gastric, and colon cancer. Prevalence in lung cancers is not well understood (Lorenzi et al. 2020).
Lynch Syndrome and MSI
Lynch syndrome, the most prevalent hereditary cancer predisposition, is caused by mutations in MMR genes. Identifying Lynch syndrome is crucial for:
- Tailoring cancer screening schedules
- Reducing cancer risk
- Improve cancer-related health outcomes
- Enable delivery of preventative treatments
- Detecting associated familial risks
To screen for Lynch syndrome, two primary methods are employed: microsatellite instability testing by PCR (MSI-PCR) and mismatch repair protein testing by immunohistochemistry (MMR by IHC). Neither method diagnoses Lynch syndrome directly, but both can indicate a deficient mismatch repair system in a tumor, potentially pointing towards the presence of Lynch syndrome and necessitating further diagnostic work.
MSI by PCR and MMR by IHC individually show high sensitivity but are not infallible. MSI by PCR may miss approximately 0.3-10% of cases, and MMR by IHC may underestimate around 5-11% of cases (Dudley et al. 2016). Combining these tests, a method called co-testing, has been shown to increase sensitivity, potentially reaching near 100% (Moreira et al. 2012). The medical community increasingly acknowledges the benefits of this dual testing approach to improve the accuracy of Lynch syndrome screening (National Comprehensive Cancer Network 2024; Stjepanovic et al. 2019).
The occasional discrepancies between MSI by PCR and MMR by IHC results can be due to factors such as retained antigenicity of mismatch repair proteins, which can affect IHC results, and tumor heterogeneity or MSI polymorphisms that can influence PCR outcomes. Co-testing mitigates these issues by providing a more comprehensive view, ensuring that tumors with MSI-high are more accurately identified.
Click here to learn more about Lynch syndrome and the importance of determining status.

"Identifying Lynch syndrome through comprehensive screening empowers patients and their healthcare providers with the knowledge to take proactive steps, from increased surveillance to preventive measures, ultimately reducing cancer burden and improving outcomes." Honey V Reddi, PhD, DABMGG, FACMG - Belay Diagnostics
Common Characteristics of MSI-H/dMMR Patients
MSI-H/dMMR tumors exhibit certain characteristics in their clinical presentation. Generally, patients with these tumors are more likely to be diagnosed at an earlier stage as opposed to a later one. There's a notable prevalence of MSI-H in older age groups, aligning with the general trend of increased cancer incidence with age. Interestingly, a deviation from this pattern occurs in younger patients, particularly those with early-onset hereditary cancers like colorectal cancer, which are often associated with genetic syndromes such as Lynch syndrome.
When we consider the incidence of MSI-H colorectal cancers across different races in the United States, studies suggest similar rates among various racial groups. This is despite the varying risks for colorectal cancer overall, where, for instance, African-American individuals are known to have a higher risk compared to other populations (Ashktorab et al. 2016).
Click here for more information on racial and ethnic diversity in MSI
Clinical Guidelines and Treatment Implications
Universal Screening Recommendations
Several clinical guideline organizations recommend MSI testing as an aid in the diagnosis of Lynch syndrome. The graphic below highlights the breadth of these recommendations globally and in the United States.

Abbreviations: AGPS – Australasian Gastrointestinal Pathology Society, AMP- Association for Molecular Pathology, ASCO- American Society for Clinical Oncology, ASCP – American Society for Clinical Pathology, CAP – College of American Pathologists, EGTM – European Group on Tumour Markers, EHTG – European Hereditary Tumour Group, ESMO – European Society for Medical Oncology, JSCO – Japan Society of Clinical Oncology, JSMO – Japanese Society of Medical Oncology, NSGC – National Society of Genetic Counselors, NICE – National Institute for Health
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric V.3.2024, all colorectal and endometrial cancers should be screened for MMR deficiency to maximize sensitivity of detection of Lynch syndrome and simplify care processes (shown on pg. LS-A, 1 of 10). This recommendation is based on several pieces of supporting peer-reviewed literature (Latham et al. 2019; Moreira et al. 2012; Mvundura et al. 2010; Pérez-Carbonell et al. 2012). Counseling by an individual with expertise in genetics is not required prior to routine tumor testing.
These NCCN Guidelines® also recommend that adenocarcinomas such as small bowel, ovarian, gastric, pancreatic, biliary tract, brain, bladder, urothelial, and adrenocortical cancers, in addition to sebaceous neoplasms, should also be considered for MMR deficiency screening, regardless of age at diagnosis. As part of a universal Lynch syndrome screening program, the NCCN Guidelines recommend molecular testing, including IHC to assess abnormal MMR protein expression and/or MSI analysis to evaluate tumors for MSI-H. Any patient whose tumor is found to be dMMR/MSI-H should be considered for subsequent evaluation for Lynch syndrome.
Further discussion and recommendations for a universal screening approach, in which all individuals newly diagnosed with colorectal cancer (CRC) have either MSI or IHC testing (shown on page MS-7). This universal screening approach provides a sensitivity of up to 100% and a specificity of 93.0% for helping identify individuals who might have Lynch syndrome (Moreira et al. 2012). A detailed description of these guideline recommendations is highlighted in the graphics below.
NCCN Guidelines
- Screening of all colorectal and endometrial cancers to maximize sensitivity for identifying individuals with Lynch syndrome and to simplify care processes. (LS-A, 1 of 10)
- Also, screening for sebaceous neoplasms and the following adenocarcinomas: small bowel, ovarian, gastric, pancreas, biliary tract, brain, bladder/urothelial, and adrenocortical cancers regardless of age at diagnosis. Counseling by an individual with expertise in genetics is not required prior to routine tumor testing. (LS-A, 1 of 10)
- Tumor testing with IHC and/or MSI as the primary approach for pathology-lab-based universal screening. (MS-9)
- Recommends use of only one test initially, either MSI or IHC. Then if normal results are obtained and Lynch syndrome is strongly suspected then the other test can be carried out. (MS-8)
- Universal screening approach provides sensitivity of 100% (95% CI, 99.3–100%) and specificity of 93.0% (95% CI, 92.0–93.7%) for identifying individuals with Lynch syndrome. (MS-7)
- MSI-H in tumors refers to the tumor having a proportion of alterations in a predetermined panel of microsatellite repeat markers that indicates the loss of MMR activity. Its significance, use, and implications are similar to that of IHC, although the tests are slightly complementary. (LS-A 4 of 10)
- Promega panel → five mononucleotide loci (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) and two pentanucleotide loci (used for specimen identification).
- Bethesda/NCI panel → two mononucleotide loci (BAT-25 and BAT-26) and three dinucleotide loci (D2S123, D5S346, and D17S250).
- Laboratories vary in their approach in testing MSI. Dinucleotide markers may be less specific than mononucleotide markers of MSI. (LS-A 4 of 10)
- MSI can be detected through bioinformatic analysis of NGS.
- Rather than 5–8 microsatellite foci analyzed (as performed in MSI by PCR), NGS can analyze anywhere from dozens to hundreds of microsatellites.
- Various comparative methods exist to identify MSI: tumor vs. paired normal or tumor vs. baseline normal. (LS-A 5 of 10)
- Further studies are needed to determine the sensitivity and specificity compared to MMR IHC and MSI by PCR.
- MSI by NGS does not require confirmation by more traditional measurement of MSI by PCR or IHC if the laboratory has validated the assay for use in the cancer in which it is being used. (LS-A 5 of 10)
For more information on specific guideline statements, please visit the specific clinical practice guideline organization or contact our Medical Affairs department at MedicalAffairs@promega.com
Global Guideline Recommendations for Performing MSI Testing
- For Lynch syndrome associated tumors, current standard is a 5 poly-A mononucleotide repeat panel or Bethesda/NCI panel can be used.
- If only dinucleotide repeats are altered, a secondary panel with mononucleotide repeats should be tested.
- Offer testing to all people with colorectal cancer, when first diagnosed, using IHC or MSI testing to identify tumours with deficient DNA mismatch repair, and to guide further sequential testing for Lynch syndrome.
- Strong recommendation for MSI testing by PCR, IHC MMR, and NGS-based testing.
- FALCO Biosystem’s MSI test (BAT-25, BAT-26, NR-21, NR24, AND MONO-27) described as approved companion diagnostic for pembrolizumab (Keytruda) and discusses mononucleotide markers detect MSI-H with high sensitivity and specificity.
ESMO: (Luchini et al. 2019) NICE: (UK National Institute for Health and Care Excellence 2017) JSMO: (Bando et al. 2024)
MSI and Treatment Decisions
Immune System Engagement in MSI-H Tumors
As discussed previously, dMMR/MSI-H has been detected across solid tumors and one hallmark feature is the notable increase in tumor infiltrating lymphocytes, which is attributed to the process of neoantigen formation. MMR deficiency leads to widespread microsatellite instability, affecting both non-coding and coding DNA regions. When instability occurs in coding regions, it often results in frame shift mutations that produce truncated, nonfunctional proteins.
These aberrant proteins are then presented as antigens to the immune system, specifically to T-cells within the tumor microenvironment. As a result, this presentation elicits the proliferation of T-cell clones that recognize these neoantigens, a response that is characteristic of MSI-H tumors. Consequently, we observe a robust infiltration of T-cells, which does not occur to the same extent in microsatellite stable (MSS) tumors.
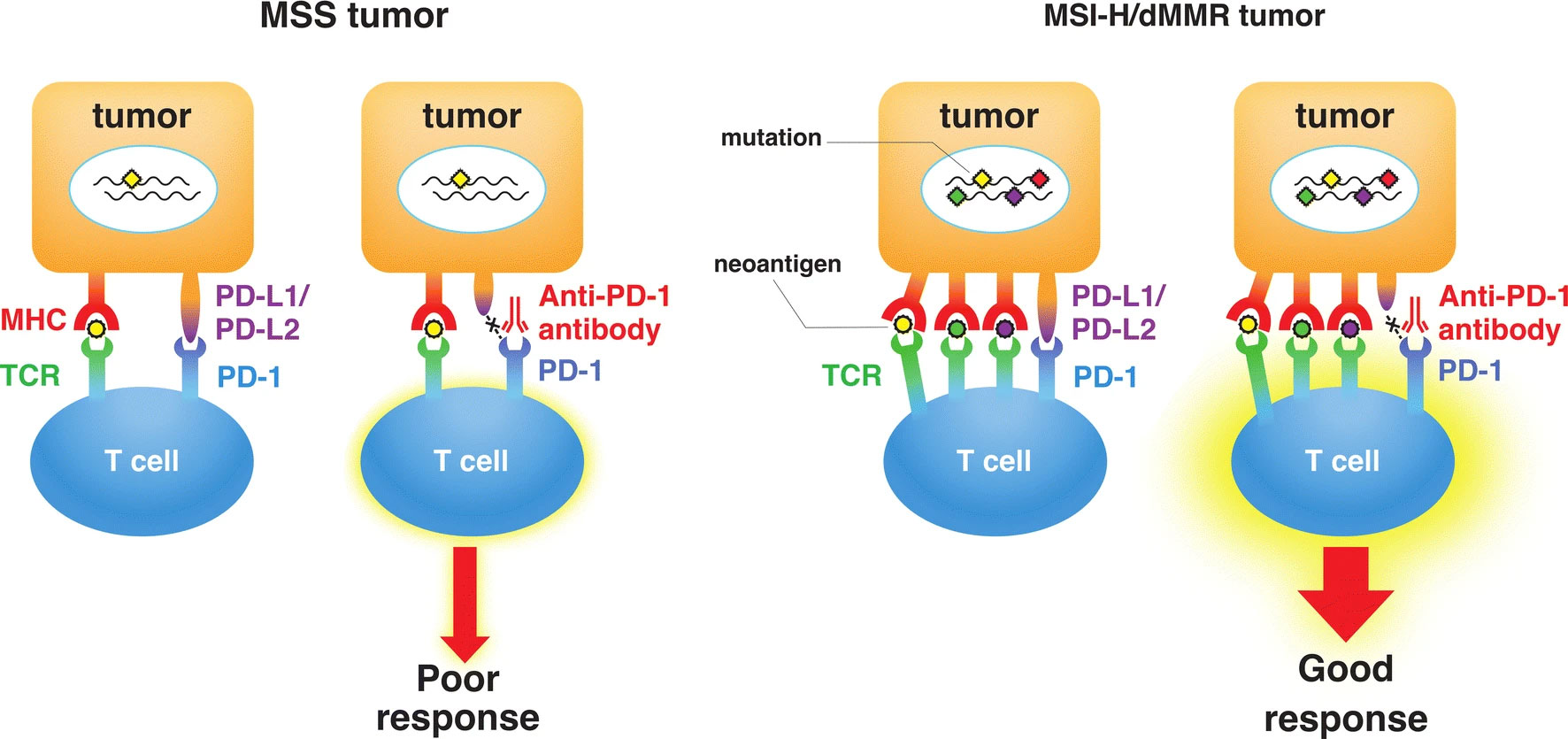
Difference in the response to immune checkpoint therapy between microsatellite-stable (MSS) tumors and microsatellite instability–high or mismatch repair deficiency (MSI-H/dMMR) tumors. High mutation burden (chrombuses) in MSI-H/dMMR tumor leads to the synthesis of mutation-associated neoantigens (small circles) presented by major histocompatibility complex (MHC) class I molecules, which attracts cytotoxic T lymphocytes to the tumor microenvironment via T cell receptor (TCR) engagement with MHC. Blockade of the programmed cell death protein 1 (PD-1)–programmed cell death ligand 1 (PD-L1) interaction with an anti–PD-1 antibody results in T cell activation and infiltration into the tumor, leading to an objective tumor response.
(Eso et al. 2020) Licensed under CC by 4.0
MSI Status and Its Impact on Cancer Treatment
MSI status significantly impacts treatment strategies. For example, patients with MSI-H colorectal cancer may not benefit from 5-fluorouracil chemotherapy (Diao et al. 2021). Moreover, these tumors frequently exhibit increased expression of immune checkpoint proteins, such as PD-L1.
In the tumor microenvironment, the interaction between PD-1 on T-cells and PD-L1 on tumor cells typically results in an 'off signal,' preventing the immune system from attacking the tumor. With the administration of anti-PD-1 or anti-PD-L1 antibodies, this inhibitory interaction is disrupted, effectively removing the 'brakes' on the immune system and enabling it to mount a robust anti-tumor response.
Consequently, MSI-high tumors, characterized by their high neoantigen load and immune cell infiltration, are often responsive to checkpoint blockade immunotherapy. This therapeutic approach has revolutionized the treatment for patients with these types of tumors, offering improved outcomes in cases where traditional therapies might not be as effective.
Treatment Options for MSS Tumors
In contrast to MSI-H/dMMR tumors, not MSI-H/MSS/pMMR tumors maintain functional mismatch repair mechanisms and typically have fewer mutations, resulting in limited immunogenicity and reduced responsiveness to immune checkpoint inhibitors as standalone treatments. However, innovative combination therapies that pair these inhibitors with other agents, such as targeted or anti-angiogenic therapies, have emerged as effective options (Guven et al. 2024) These new combination therapies have shown the potential to improve survival and delay disease progression in advanced cancers, emphasizing the importance of determining MMR status to guide treatment decisions and optimize outcomes for patients with Not MSI-H/MSS/pMMR tumors.
Importance of Accurate MSI Testing for Treatment Decisions
A key study by Cohen et al. in 2018 brought to light the crucial role of co-testing for MMR and MSI (Cohen et al. 2018). The study found instances of misdiagnosis and subsequent therapeutic resistance when only IHC or MSI by PCR was used individually. Their work underlined the necessity of routinely testing for microsatellite instability or mismatch repair deficiency before administering immune checkpoint inhibitors.
As a result of these findings, the European Society of Medical Oncology (ESMO) updated their recommendations to include co-testing using both IHC and PCR (Luchini et al. 2019). This dual-testing approach ensures a more accurate identification of MSI and dMMR status in patients with advanced cancer, optimizing their eligibility for immunotherapy.
Click here for more information on how MSI status can impact treatment decisions.
Conclusion
Microsatellite instability (MSI) plays a pivotal role in cancer diagnostics, treatment decisions, and hereditary risk assessment. As our understanding of MSI continues to evolve, so does its clinical relevance, influencing everything from screening recommendations to immunotherapy eligibility. The integration of MSI testing into routine oncology practice highlights its importance as a biomarker for personalized treatment strategies. As advancements in detection methods improve sensitivity and accessibility, MSI testing will remain at the forefront of precision medicine, guiding clinicians in optimizing patient care.
For more information on MSI-related education, visit our MSI Resource Center.
References
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric V.3.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed February 13, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Ashktorab, H., Ahuja, S., Kannan, L., et al. 2016, Oncotarget, 7, 34546,
https://www.ncbi.nlm.nih.gov/pubmed/27120810
Bacher, J. W., Clipson, L., Steffen, L. S., & B. Halberg, R. 2016, Microsatellite Markers,
https://www.intechopen.com/chapters/52508
Bando, H., Yamaguchi, K., Mitani, S., et al. 2024, Cancer Sci, 115, 1014,
https://pubmed.ncbi.nlm.nih.gov/38263580/
Cohen, R., Hain, E., Buhard, O., et al. 2018, JAMA Oncol, 5, 551,
https://www.ncbi.nlm.nih.gov/pubmed/30452494
Diao, Z., Han, Y., Chen, Y., Zhang, R., & Li, J. 2021, Crit Rev OncolHematol, 157, 103171,
https://www.ncbi.nlm.nih.gov/pubmed/33290824
Dudley, J. C., Lin, M.-T., Le, D. T., & Eshleman, J. R. 2016, Clin Cancer Res, 22, 813,
https://www.ncbi.nlm.nih.gov/pubmed/26880610
Eso, Y., Shimizu, T., Takeda, H., Takai, A., & Marusawa, H. 2020, J Gastroenterol, 55, 15,
https://www.ncbi.nlm.nih.gov/pubmed/31494725
Evrard, C., Tachon, G., Randrian, V., Karayan-Tapon, L., & Tougeron, D. 2019, Cancers, 11, 1567,
https://www.ncbi.nlm.nih.gov/pubmed/31618962
Gilson, P., Merlin, J.-L., & Harlé, A. 2021, Cancers, 13, 1491,
https://www.ncbi.nlm.nih.gov/pubmed/33804907
Guven, D. C., Kavgaci, G., Erul, E., et al. 2024, Oncol, 29, e580
https://pubmed.ncbi.nlm.nih.gov/38309719/
Lorenzi, M., Amonkar, M., Zhang, J., Mehta, S., & Liaw, K.-L. 2020, J Oncol, 2020, 1,
https://doi.org/10.1155/2020/1807929
Luchini, C., Bibeau, F., Ligtenberg, M. J. L., et al. 2019, Ann Oncol, 30, 1232,
https://www.ncbi.nlm.nih.gov/pubmed/31056702
Mao, R., Krautscheid, P., Graham, R. P., et al. 2021, Genet Med, 23, 1807,
https://www.ncbi.nlm.nih.gov/pubmed/34140662
McCarthy, A. J., Capo‐Chichi, J., Spence, T., et al. 2019, J Pathol: Clin Res, 5, 115,
https://www.ncbi.nlm.nih.gov/pubmed/30387329
Mei, W.-J., Mi, M., Qian, J., et al. 2022, Front Immunol, 13, 1019582
https://pubmed.ncbi.nlm.nih.gov/36618386/
National Comprehensive Cancer Network. 2024, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric (Version 32024), https://www.nccn.org
Stjepanovic, N., Moreira, L., Carneiro, F., et al. 2019, Ann Oncol, 30, 1558,
https://www.ncbi.nlm.nih.gov/pubmed/31378807
UK National Institute for Health and Care Excellence. 2017,
https://www.nice.org.uk/guidance/dg27
Umar, A., Boland, C. R., Terdiman, J. P., et al. 2004, J Natl Cancer Inst, 96, 261,
https://www.ncbi.nlm.nih.gov/pubmed/14970275
Yamamoto, H., & Imai, K. 2019, Semin Oncol, 46, 261,
https://www.ncbi.nlm.nih.gov/pubmed/31537299
Disclosures and Acknowledgements
Honey Reddy, PhD is a member of Promega’s KOL Clinical Community Engagement Program.
Kara Chamberlin, PhD, and Annette Burkhouse, PhD, contributed content and editing of the article drafts.
Continuing Education Credit
Promega is approved as a provider of continuing education programs in the clinical laboratory sciences by the ASCLS P.A.C.E.® Program.
Promega is also a premier partner of CE Broker, the official tracking system of the Florida Board of Clinical Laboratory Personnel.
Sign up now to receive continuing education credit for this program.