Antibody Internalization Bioassay
Quantify and Monitor Antibody Internalization to Screen for Optimal Antibody Designs
- Antigen-Specific Internalization: Data accurately reflect antigen expression and ADC binding dynamics
- Payload Agnostic: ADC payload does not interfere with anti-Fc binding to target antibody
- Broad Utility: Technology works with both adherent and suspension cell backgrounds
Antibody Internalization Bioassay
| Catalog No. | Product Name | Size | Price | Qty | |||
|---|---|---|---|---|---|---|---|
| CS372004 | Early Access Antibody Internalization Bioassay | View Specifications | 1 each | Please Enquire | |||
| CS372008 | Early Access Antibody Internalization Bioassay 10X | View Specifications | 1 each | Please Enquire |
Early Access = This product is available under our Early Access program - Learn More
Catalog (FT) = This product is available under our Catalog (FT) program - Learn More
Go from Antibody Binding to Payload Delivery with Confidence
Antibody-drug conjugates (ADCs) combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs to selectively kill cancer cells. To be effective, ADCs must be internalized and trafficked inside the cell to release their payload and avoid off-target effects. Understanding internalization dynamics early during development helps researchers identify the most promising candidates, avoid costly downstream failures and scale production faster.
Traditional methods to measure antibody internalization face several limitations:
- Time-consuming flow cytometry protocols
- pH-dependent dye conjugates that alter antibody behavior
- Low-throughput fluorescence microscopy assays
What does using the Antibody Internalization Bioassay mean for you?
- Bridge the gap between binding affinity and therapeutic cytotoxicity with reproducible internalization measurements.
- Screen large panels of antibodies and conjugates for internalization efficiency in days, not weeks.
- Remove development risk by eliminating low-internalizing candidates early and avoiding off-target toxicity.
How Does the Antibody Internalization Bioassay Work?

Assay Principle
The Antibody Internalization Bioassay gives researchers an endpoint readout to monitor antibody internalization. The assay is based on an anti-Fc Fab labeled with NanoLuc® luciferase binding to a primary antibody or ADC therapeutic. When the primary antibody binds to the target antigen, the complex is internalized, while unbound antibody is washed away. The detection reagent that includes a membrane-impermeable NanoLuc® inhibitor (Bio-Glo-NL™ Glo-Guard), a membrane-impermeable NanoLuc® inhibitor buffer (Bio-Glo-NL™ Glo-Guard Buffer, yellow shading in figure) and a membrane-permeable luciferase substrate is added to the cells. The resulting luminescent signal is primarily generated from the internalized Fab:primary antibody complex.
What is the Antibody Internalization Bioassay Workflow?
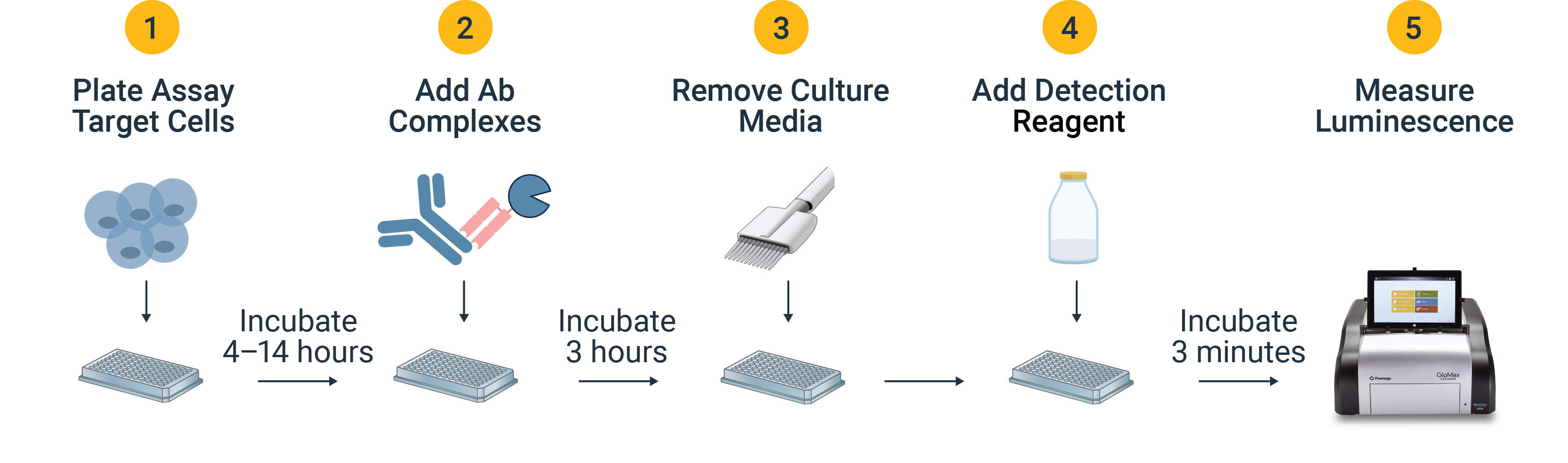
Antibody Internalization Bioassay Design
- Plate assay target cells at desired density.
- Pre-complexed antibodies are incubated with target cells, allowing target antigen binding on the cell surface and internalization.
- After incubation, media is withdrawn to remove unbound antibody so the remaining cell-associated antibodies are surface-found or internalized.
- Detection reagent that includes Bio-Glo-NB™ Live-Cell Assay Substrate, Bio-Glo-NL™ Glo-Guard and Bio-Glo-NL™ Glo-Guard Buffer is added to the wells.
- The substrate diffuses through the plasma and endosomal membranes to bind intracellular NanoLuc® luciferase, while non-internalized NanoLuc® luciferase is inhibited such that the luminescent signal is proportional to the amount of internalized primary antibody.
Accurately Compare Antibody Internalization Across Structural Variants


The Antibody Internalization Bioassay was used to quantify the internalization of two classes of ADCs and a non-conjugated version. Panel A. Raji target cells were plated with CD19-specific antibodies loncastuximab, a loncastuximab ADC and a control IgG. Panel B. SKOV3 target cells were plated with HER2-specific trastuzumab, a trastuzumab ADC and a control IgG. The Antibody Internalization Bioassay is amenable to measuring internalization in both suspension (Raji) and adherent (SKOV3) cell types.
Interested in furthering your ADC characterization with readouts like payload cytotoxicity or DAR determination?
We offer a wide range of analytical tools for toxicity, structure and specificity of your ADCs.
Protocols
No protocols available
Specifications
Catalog Number:
What's in the box?
| Item | Part # | Size |
|---|---|---|
NanoLuc® Anti-Human mAb Binder |
CS372001 | 1 × 28μl |
Bio-Glo-NL™ Glo-Guard Buffer |
CS372002 | 1 × 3ml |
Bio-Glo-NL™ Glo-Guard |
CS372003 | 1 × 17μl |
Bio-Glo-NB™ Live-Cell Luciferase Assay Substrate |
JB120A | 1 × 0.125ml |
What's in the box?
| Item | Part # | Size |
|---|---|---|
NanoLuc® Anti-Human mAb Binder |
CS372005 | 1 × 280μl |
Bio-Glo-NL™ Glo-Guard Buffer |
CS372006 | 1 × 30ml |
Bio-Glo-NL™ Glo-Guard |
CS372007 | 1 × 170μl |
Bio-Glo-NB™ Live-Cell Luciferase Assay Substrate |
JB120B | 1 × 1.25ml |
Resources
Featured Resource
Poster: Bioluminescent Assays for Antibody-Drug Conjugate Development: Internalization and Bystander Killing Functions
Antibody-drug conjugates (ADCs) combine targeted delivery with potent cytotoxic payloads, utilizing multiple mechanisms of action (MoA). These include direct cytotoxicity following internalization of the ADC by antigen-expressing cells, killing of bystander cells through cleaved payloads, antigen function blockade and immune-mediated cytotoxicity (e.g., ADCC and ADCP) driven by the antibody Fc region. To address the need to capture MoA of ADCs, we developed bioluminescent tools to streamline ADC evaluation, focusing on antibody internalization and bystander killing.

Related Products
Similar Products
pHAb Reactive Dyes
Measure antibody internalization in real time with pH-sensitive fluorescent dyes.
G9841, G9831, G9835, G9845
Magne® Protein G and Magne® Protein A Beads
Magnetic affinity beads with Protein A or G for Ab purification from culture media, ascites and serum samples.
G7471, G7472, G7473, G8781, G8782, G8783
Raji (HiBiT) TCK Bioassay
Simple, fast and sensitive technology to specifically measure target cell killing.
JA1211, JA1215, M2452, GA6050, CS3055A14, CS3055A17, CS3055B16, CS3055B17, JA1251, JA1255, JA1261, JA1265, JA1271, JA1275, CS3055C09, CS3055C10
Lumit® FcγR Binding Immunoassays
Novel homogeneous competition assays to measure the interaction between human Fc receptors and antibodies or Fc fusion proteins.
W7030, W7031, W7040, W7041, W7050, W7051, W7060, W7061, W7070, W7071, W7080, W7081
Frequently Used With
CytoTox-Glo™ Cytotoxicity Assay
Highly sensitive luminescent cytotoxicity assay that measures the relative number of dead cells.
G9290, G9291, G9292
Lumit® FcγR Binding Immunoassays
Novel homogeneous competition assays to measure the interaction between human Fc receptors and antibodies or Fc fusion proteins.
W7030, W7031, W7040, W7041, W7050, W7051, W7060, W7061, W7070, W7071, W7080, W7081
ADCC Reporter Bioassay, V Variant
Measure potency and stability of antibodies mediating ADCC through the high-affinity human FcγRIIIa-V receptor.
G7015, G7014, G7010, G7016, G7013, G7018, G7102, GA1130
Trypsin Platinum, Mass Spectrometry Grade
Maximum digest specificity. Maximum resistance to autoproteolysis.
VA9000



