OncoMate® MSI Dx Analysis System
Now FDA-Approved Companion Diagnostic in the US. The OncoMate® MSI Dx Analysis System is indicated for use as a companion diagnostic test to identify patients with microsatellite stable (MSS; defined as not MSI-high [not MSI-H]) endometrial carcinoma who may benefit from treatment with KEYTRUDA® (pembrolizumab) in combination with LENVIMA® (lenvatinib) in accordance with the approved therapeutic product labeling.
- IVD for Microsatellite Instability Characterization
- First and only MSI by PCR test FDA-approved in the US for companion diagnostic use in endometrial carcinoma for use in your lab
- Fragment sizing test used to determine MSI status with Gold Standard microsatellite markers
- FDA-approved in the United States; CE-marked and available in select European countries
Catalog Number:
Size
Catalog Number: MD2140
Accurately Determine MSI Status Using the Gold Standard Method
The OncoMate® MSI Dx Analysis System is a fluorescent, multiplex PCR-based test to detect microsatellite instability (MSI) status. MSI is a form of genomic instability caused by the deletion of repeating bases within microsatellites during DNA replication due to the failure of the mismatch repair system (MMR) to correct these errors. The OncoMate® MSI Dx Analysis System is designed to provide physicians with a functional, molecular measurement of the level of DNA mismatch repair deficiency demonstrated within their patient’s tumor.
With FDA approval as a companion diagnostic, the OncoMate® MSI Dx Analysis System now provides clinicians with a validated tool to identify patients with MSS (not MSI-H) endometrial carcinoma who may benefit from combination therapy options.
The OncoMate® MSI Dx Analysis System is FDA-cleared as an aid to identify candidates for further testing for Lynch Syndrome with colorectal cancers.
The FDA-approved OncoMate® MSI Dx Analysis System (Cat.# MD2140) is available in the United States, American Samoa, Guam, Puerto Rico, and the US Virgin Islands. For information about availability in other regions, including the CE-marked system available in select European countries, please contact us.
MSI Testing Empowers Precision Medicine Through Actionable Insight
Uncover Hereditary Risk and Guide Confident Diagnostic and Management Decisions in Colorectal Cancer
Testing colorectal cancers for microsatellite instability (MSI) is critical for identifying patients with Lynch syndrome, enabling early detection of new or recurrent cancers in patients and their families. MSI-H status indicates defects in the DNA mismatch repair (MMR) system, typically caused by autosomal dominant mutations in MMR genes. PCR-based MSI testing provides clear evidence of MMR function, helping clinicians assess hereditary cancer risk and guide appropriate management.
Inform Effective Immunotherapy Decisions With MSI Testing in Endometrial Cancer
Testing endometrial cancers for microsatellite instability (MSI) helps ensure each patient receives the most appropriate treatment by revealing molecular characteristics of the cancer and the tumor environment. MSI status informs immunotherapy eligibility for endometrial cancer, including MSS cases, which comprise roughly 70% of endometrial cancers.
The OncoMate® MSI Dx Analysis System, an FDA-approved companion diagnostic, delivers high analytical precision to ensure that MSS results truly reflect microsatellite stability rather than a missed MSI-H finding, and supports informed, confident treatment decisions.
Performance and Methodology To Meet Clinical Demands
Only Two Sections Required

FFPE sections with a tissue volume of 0.1mm3 to 2.0mm3. Tumor sample should have >20% viable tumor cells.
Minimal DNA Required

Uses only 1ng of amplification-quality DNA per reaction.
Assay Time Under 3 Hours

Quick, multiplexed reaction, balanced for accuracy and efficiency, goes from DNA to answer in as little as 2.5 hours.
Sensitive and Specific Results

Reliable MSI determination in colorectal cancers with strong agreement with IHC.
Only Two Sections Required

From FFPE tumor samples and blood or FFPE normal samples.
Minimal DNA Required

Uses only 1ng of amplification-quality DNA per reaction.
Assay Time Under 3 Hours

Quick, multiplexed reaction, balanced for accuracy and efficiency, goes from DNA to answer in as little as 2.5 hours.
Sensitive and Specific Results

Accurate MSI determination in endometrial carcinoma with strong agreement with IHC.
Demonstrated clinical performance In the subset of KEYNOTE-775 participants with MSS (not MSI-H) tumor status by the OncoMate® MSI Dx Analysis System, pembrolizumab plus lenvatinib efficacy was comparable to the efficacy in all clinical trial assay pMMR (CTA-pMMR) participants randomized in the trial.
Sensitive Panel of Mononucleotide Repeat Markers
The OncoMate® MSI Dx Analysis System targets five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) that were selected for high sensitivity and specificity to alterations in repeat lengths in samples containing mismatch repair defects1,2. For quality control and sample authentication of matched tumor and normal samples, the system uses two pentanucleotide repeat markers (Penta C and Penta D) that were selected for their high level of polymorphism and lower degree of MSI. In a study of colorectal cancers, the OncoMate® MSI Dx Analysis System showed concordance with immunohistochemistry (97.8% PPA)3. In a study using endometrial carcinoma, the OncoMate® MSI Dx Analysis System showed 99.0% agreement between MSS (not MSI-H) (OncoMate) and pMMR (by IHC) results3.
Test Design and Marker Information
Target Markers

NCI recommended, Revised Bethesda Panel and two pentanucleotide markers4.
Example Data
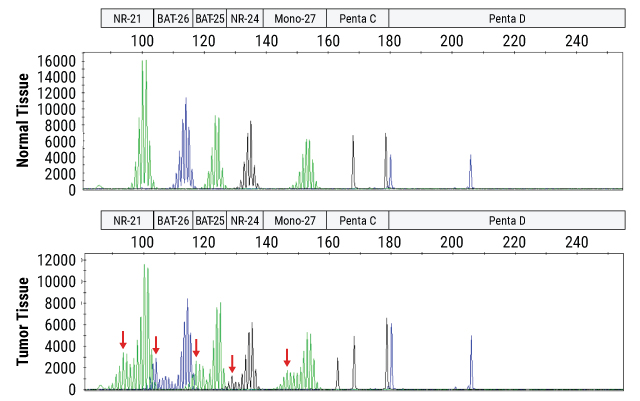
Instability is determined by fragment size analysis on a capillary electrophoresis instrument following PCR amplification of DNA from a patient's normal and tumor tissue samples. Unstable loci in the tumor tissue are indicated by red arrows.
Sample to Answer Overnight
The OncoMate® MSI Dx Analysis System is part of a broader workflow that includes DNA extraction from FFPE tissue samples, quantitation of DNA, amplification of specific microsatellite markers using multiplex PCR, fragment separation by capillary electrophoresis, and data analysis and interpretation.

Isolate DNA
from FFPE samples using the Maxwell® CSC DNA FFPE Kit and the Maxwell® CSC Instrument

Quantitate DNA
using fluorescent DNA quanitation reagents (e.g., Quantifluor® Dx dsDNA System) and instruments

Amplify DNA
using the OncoMate® MSI Dx Analysis System

Calibrate the Dye Spectrum
using the OncoMate® 5C Matrix Standards

Separate and Detect Fragments
using the Applied Biosystems® 3500 Dx Genetic Analyzer

Data Analysis and Reporting
using the OncoMate® MSI Dx Interpretive Software

Turnaround Time:
In as little as 10 hours or overnight

The OncoMate® MSI Dx Analysis System for use as a companion diagnostic is part of a broader workflow that includes DNA extraction from FFPE tissue samples, quantitation of DNA, amplification of specific microsatellite markers using multiplex PCR, fragment separation by capillary electrophoresis, and data analysis and interpretation.

Isolate DNA
From FFPE tumor samples and blood or FFPE normal samples using nucleic acid extraction reagents and instruments

Quantitate DNA
using fluorescent DNA quanitation reagents (e.g., Quantifluor® Dx dsDNA System) and instruments

Amplify DNA
using the OncoMate® MSI Dx Analysis System

Calibrate the Dye Spectrum
using the OncoMate® 5C Matrix Standards

Separate and Detect Fragments
using the Applied Biosystems® 3500 Dx Genetic Analyzer

Data Analysis and Reporting
using the OncoMate® MSI Dx Analysis Software

Turnaround Time:
In as little as 10 hours or overnight

Automated Software for Easy MSI Analysis
Capillary electrophoresis data requires further analysis to assign marker and size information. We have developed an automated MSI interpretive analysis software that summarizes critical data features and provides an automated MSI status determination.
Intended use statement for use as a companion diagnostic test to identify patients with endometrial carcinoma who may benefit from treatment with KEYTRUDA® (pembrolizumab) in combination with LENVIMA® (lenvatinib)
The OncoMate® MSI Dx Analysis System is a qualitative multiplex polymerase chain reaction (PCR) test intended to detect the deletion of mononucleotides in five microsatellite loci (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) for the identification of microsatellite instability (MSI) using DNA obtained from formalin-fixed, paraffin-embedded (FFPE) endometrial carcinoma tissue specimens, and DNA isolated from matched normal FFPE specimen or whole blood. The OncoMate® MSI Dx Analysis System is for use with the Applied Biosystems® 3500 Dx Genetic Analyzer and OncoMate® MSI Dx Analysis Software.
The OncoMate® MSI Dx Analysis System is indicated for use as a companion diagnostic test to identify patients with microsatellite stable (MSS; defined as not MSI-high [not MSI-H]) endometrial carcinoma who may benefit from treatment with KEYTRUDA® (pembrolizumab) in combination with LENVIMA® (lenvatinib) in accordance with the approved therapeutic product labeling.
Intended use statement for use as an aid to identify candidates for further testing for Lynch Syndrome with colorectal cancers
The OncoMate® MSI Dx Analysis System is a qualitative multiplex polymerase chain reaction (PCR) test intended to detect the deletion of mononucleotides in five microsatellite loci (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) using matched tumor and normal DNA obtained from formalin fixed, paraffin-embedded (FFPE) colorectal tissue sections. The OncoMate® MSI Dx Analysis System is for use with the Applied Biosystems® 3500 Dx Genetic Analyzer and OncoMate® MSI Dx Interpretive Software.
The OncoMate® MSI Dx Analysis System is indicated in patients diagnosed with colorectal cancer (CRC) to detect microsatellite instability (MSI) as an aid in the identification of probable Lynch syndrome to help identify patients that would benefit from additional genetic testing to diagnose Lynch syndrome. Results from the OncoMate® MSI Dx Analysis System should be interpreted by healthcare professionals in conjunction with other clinical findings, family history, and other laboratory data.
Important MSI publications for Endometrial Cancer and Immune Checkpoint Inhibitors
MSI is a well-accepted, predictive, tissue-agnostic biomarker that predict response to immune checkpoint inhibitors (ICIs). Since MSI can affect a wide range of genes, it can lead to an increase in mutations and development of neoantigens. Because of this, MSI-H tumors typically show high levels of immune cell infiltration and, conversely, MSS tumors usually have low infiltration. ICIs and combination therapies work within the tumor environment to encourage the immune system to combat the cancer more effectively. Durable responses have been observed in MSI-H patients treated with ICIs as well as in MSS (not MSI-H) endometrial cancers with combination treatments.
Baretti, M., and Le, D.T. (2018) DNA mismatch repair in cancer. Pharmacol. Ther. 189, 45–62.
Johannet, P. et al. (2025) Therapeutic targeting of mismatch repair-deficient cancers. Nat. Rev. Clin. Oncol. 22(10) ,734–759.
Le, D.T. et al. (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–13.
Arora, S. et al. (2020) FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin Cancer Res. 26(19), 5062–7.
Makker, V. et al. (2023) Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: Updated efficacy and safety from the randomized Phase III study 309/KEYNOTE-775. J. Clin. Oncol. 41, 2904–10.
André, T. et al. (2023) Antitumor activity and safety of dostarlimab monotherapy in patients with mismatch repair deficient solid tumors: A nonrandomized controlled trial. JAMA Netw Open 6(11), e2341165.
Important MSI Publications and Guidelines for Colorectal Cancer Cancer and Lynch Syndrom
Microsatellite instability resulting from deficiencies in DNA mismatch repair (MMR) can occur due to mutations (sporadic or germline) or hypermethylation of MMR genes. These changes disrupt expression of functional MMR proteins, allowing replication errors to accumulate across the genome. Global genomic mutations disrupt normal cellular function, which leads to unchecked growth and cancers but also produces novel proteins. Instability, or mutations, of mononucleotide repeat microsatellite sequences are particularly sensitive to replication errors and can be the first evidence of MMR deficiency. Individuals with Lynch syndrome, the primary hereditary tumor syndrome associated with MSI-H, have an inherited defect which disrupts normal MMR function, making these individuals predisposed for the development of certain cancers, including colorectal cancers. MSI status has a long history of aiding in the identification of patients who may benefit from additional genetic testing to identify whether or not they are a Lynch syndrome carrier.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)5.
Stjepanovic, N. et al. (2019) Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology 30, 1558-1571, doi:10.1093/annonc/mdz233 (2019).
Bacher, J. W. et al. (2004) Development of a fluorescent multiplex assay for detection of MSI-High tumors. Dis Markers 20, 237-250, doi:10.1155/2004/136734.
Boland, C. R. and Goel, A. (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138, 2073-2087.e2073, doi:10.1053/j.gastro.2009.12.064.
Umar, A. et al. (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. Journal of the National Cancer Institute 96, 261-268, doi:10.1093/jnci/djh034.
Rubenstein, J. H. et al. (2015) American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology 149, 777-782; quiz e716-777, doi:10.1053/j.gastro.2015.07.036.
Herzig, D. O. et al. (2017) Clinical Practice Guidelines for the Surgical Treatment of Patients With Lynch Syndrome. Dis Colon Rectum 60, 137-143, doi:10.1097/DCR.0000000000000785.
Stoffel, E. M. et al. (2015) Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 33, 209-217, doi:10.1200/JCO.2014.58.1322.
Sepulveda, A. R. et al. (2017) Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. Archives of Pathology & Laboratory Medicine 141, 625-657, doi:10.5858/arpa.2016-0554-CP.
Hampel, H. et al. (2015) A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17, 70-87, doi:10.1038/gim.2014.147.
Boardman, L. A. et al. (2020) AGA Clinical Practice Update on Young Adult-Onset Colorectal Cancer Diagnosis and Management: Expert Review. Clin Gastroenterol Hepatol 18, 2415-2424, doi:10.1016/j.cgh.2020.05.058.
References
- Bacher, J. et al. (2004) Dis. Markers 20, 237.
- Luchini, C. et al. (2019) Annals of Oncology. 30, 1232.
- OncoMate® MSI Dx Analysis System Technical Manual #TM543 (2025) Promega Corporation.
- Umar, A. et al. (2004) J. Natl. Cancer Inst. 96, 261–8.
- Referenced with the permission from NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric V1. 2025 © National Comprehensive Cancer Network, Inc., 2025. All rights reserved. Accessed November 10, 2025. To view most recent and complete version of the guideline, go online to nccn.org.
NCCN makes no warranties of any kind whatsoever regarding the content, use or application and disclaims any responsibility for their application or use in any way.
Seamless Support for Adopting OncoMate® MSI Dx in Your Lab
Our team is dedicated to partnering with you to ensure success and has broad experience training laboratories. We offer an online user training course as well as virtual and in-person training to get your lab up and running with the OncoMate® MSI Dx Analysis System more efficiently.

Medical Information
If you need assistance or access to medical information and resources, our Medical Affairs scientists are ready to help.
Protocols
Specifications
Catalog Number:
What's in the box?
| Item | Part # | Size | Concentration |
|---|---|---|---|
Water, Amplification Grade |
MD193A | 1 × 1,250μl | |
OncoMate® MSI 5X Master Mix |
MD280A | 1 × 200μl | |
Size Standard 500 |
MD500A | 1 × 100μl | |
OncoMate® MSI 5X Primer Mix |
MD705A | 1 × 200μl | |
2800M Control DNA |
MD810A | 1 × 25μl | 10ng/μl |
SDS
Search for SDSCertificate of Analysis
Use Restrictions
For In Vitro Diagnostic Use. This product is only available in certain countries.Storage Conditions
U.S. Pat. No. 9,139,868, European Pat. No. 2972229, Japanese Pat. No. 6367307 and other patents pending.
TMR-ET, CXR-ET and WEN dyes are proprietary.
Related Products
Frequently Used With
OncoMate® 5C Matrix Standard
Used to calibrate capillary electrophoresis instruments prior to running the OncoMate® MSI Dx Analysis System.
MD4850
OncoMate® MSI Dx Interpretive Software
Used to generate automated MSI results with data generated by Applied Biosystems® 3500 Dx Genetic Analyzer using OncoMate® MSI Dx Analysis System amplification products.
MD4140
QuantiFluor® Dx dsDNA System
Fluorescent DNA-binding dye that enables sensitive quantification of small amounts of double-stranded DNA (dsDNA) in a purified sample. For In Vitro Diagnostic Use.
E5900
Maxwell® CSC Instrument
Benchtop instrument for automated nucleic acid purification from a range of clinical sample types. For In Vitro Diagnostic Use.
AS6000
Maxwell® CSC DNA FFPE Kit
Isolates DNA from FFPE samples for in vitro diagnostic assays using the Maxwell® CSC Instrument.
AS1350
Maxwell® CSC Blood DNA Kit
Isolates DNA from blood for in vitro diagnostic assays using the Maxwell® CSC Instrument.
AS1321
Maxwell® CSC 48 Instrument
Benchtop instrument for automated nucleic acid purification from a range of clinical sample types. For In Vitro Diagnostic Use.
AS8000

