RNasin® Plus RNase Inhibitor: New Protein for High Temperature RNase Inhibition
Promega Corporation
Publication Date: 2003
Abstract
RNasin Plus RNase Inhibitor inhibits ribonucleases during reaction assembly. Heating of these reactions to as high as 70°C for 15 minutes does not result in reappearance of ribonuclease activity. Thus RNasin® Plus RNase Inhibitor can be added prior to RT-PCR to protect RNA during the reverse transcription step.
Introduction
Ribonucleases (RNases) are the bane of RNA-based research, and innumerable publications have expounded on the need to create an RNase-free environment for successful RNA work. RNase A, however, is a common tool used in molecular biology laboratories and is most prominently used in plasmid purification protocols to remove bacterial RNA from plasmid DNA. RNA purification methods are absolutely geared to produce RNase-free RNA, but assembly of reactions in such an environment may introduce exogenous RNases. Pipets may be used for both plasmid work and RT-PCR reaction set-up and become a source of exogenous RNases. Even robotic liquid handlers used as multipurpose laboratory tools, at one time performing plasmid purifications and at another purifying RNA and assembling RT-PCR reactions, could become a source of RNase contamination. Promega RNasin® Ribonuclease Inhibitor has been a major source of comfort for molecular biology researchers. The protein binds to RNases like RNase A and protects your RNA sample from degradation (1) (2) . The inhibitor holds onto the RNase during reaction assembly and preserves your sample.
Strong secondary structure of some RNAs is often a problem, and techniques developed to handle difficult secondary structure are generally considered out of the useful temperature range for RNase inhibition. RNases are notoriously heat stable. In fact, a common method for producing DNase-free RNase A requires the user to boil the protein sample and let the protein renature (3) . One method of reducing secondary structure is to heat denature the RNA template prior to RT-PCR and/or perform the reverse transcription (RT) reaction at an elevated temperature. Heat denaturation usually involves heating the RNA sample at 65–70°C for 5–10 minutes then quick cool the RNA on ice to prevent renaturation of the secondary structure (4) (5) . This denatured template is then used to assemble the RT reaction. To further ensure successful reverse transcription through any secondary structure, reactions can be performed at temperatures ≥50°C (4) . More and more enzymes are capable of performing reverse transcription well at these elevated temperatures including AMV Reverse Transcriptase (Cat.# M5101), ImProm-II™ Reverse Transcriptase (Cat.# A3802) (4) and Tth DNA Polymerase (Cat.# M2101) (6) . RNasin®-style inhibitors have traditionally been left out of these reactions due to the unstable nature of the protein at these temperatures. The new RNasin® Plus RNase Inhibitor can be added to the RNA sample prior to thermal denaturation.
RNasin® Plus RNase Inhibitor is a new recombinant mammalian RNase inhibitor capable of inhibiting eukaryotic RNases, such as RNase A and RNase B, similar to the human version of the protein. Once the inhibitor and an RNase like RNase A are combined, the solution can be heated to temperatures as high as 70°C for 15 minutes, and the RNase activity does not reappear (Figure 1). RNasin® Plus has been tested in RT-PCR and, like the human protein, is compatible with enzymes like AMV, M-MLV or ImProm-II™ Reverse Transcriptases as well as Taq and Tfl DNA Polymerases. RNasin® Plus has also been tested and found to be compatible with quantitative real-time RT-PCR in a TaqMan® Assay. The new protein can even preserve templates from degradation in a biological extract as complex as a rat liver protein extract, demonstrating a broad spectrum of RNase inhibition. Again, this inhibition is stable at a temperature of 70°C for 15 minutes (Figure 2).
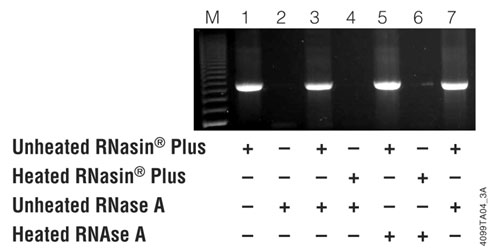
Figure 1. RNasin Plus inhibits RNase A and protects a template prior to RT-PCR. One microgram of an RNA template was combined with 1μl of unheated RNasin® Plus (40 units), 2μl of unheated RNase A (20ng), 1μl of heated RNasin® Plus or 2μl heated RNase A as indicated in the figure. For lane 7, 1μl of unheated RNasin® Plus and 2μl of unheated RNase A were combined then heated together at 70°C for 15 minutes. Five microliters of the assembled reactions was then used as template for RT-PCR using the AccessQuick™ RT-PCR System and luciferase control RNA-specific primers as directed in the AccessQuick™ RT-PCR System Product Information Sheet, 9PIA170. The RT step was performed at 48°C for 45 minutes, and the reaction was heated at 96°C for 2 minutes. Twenty cycles of 15 seconds denaturation (95°C); 60 second hybridization (55°C) and 90 second extension (72°C) was followed by a 5 minute final extension. Fifteen microliters of the reaction was analyzed by agarose gel electrophoresis with ethidium bromide staining. Lane M is the 200bp DNA Step Ladder (Cat.# G6961).
Figure 2. RNasin® Plus inhibits RNases in a rat liver protein extract and protects a template prior to RT-PCR. One hundred nanograms (lanes 1–7) or 10ng (lanes 8–14) of an RNA template was combined with 20μl of unheated RNasin® Plus, 2.5μl of unheated extract, 20μl heated RNasin® Plus or 2.5μl heated extract as indicated in the figure. For lanes 7 and 14, 20μl of unheated RNasin® Plus and 2.5μl of unheated extract were combined then heated together at 70°C for 15 minutes. Each sample was incubated at 37°C for 1 hour. Five microliters of the assembled reactions was then used as template for RT-PCR as described in Figure 1.
Heating RNasin® Plus Solutions Does Not Result In Reappearance of RNase A Activity
To demonstrate that RNase A is not reactivated when heated in the presence of RNasin® Plus, the following five solutions were prepared:
1. RNasin® Plus (40u/µl) heated at 70°C for 15 minutes.
2. RNasin® Plus (40u/µl) kept on ice.
3. RNase A (10µg/ml) heated at 70°C for 15 minutes.
4. RNase A (10µg/ml) kept on ice.
5. 17µl water, 1µl RNasin® Plus (40 units) and 2µl of RNase A (20ng) assembled at ambient room temperature then heated at 70°C for 15 minutes.
The above solutions were used to assemble various reactions with 1µl of heated or unheated RNasin® Plus and 2µl of heated or unheated RNase A in a total volume of 20µl, as shown in Figure 1. One reaction (Figure 1, lane 7) contained the mixture of RNasin® Plus and RNase A that was assembled at room temperature and heated to 70°C for 15 minutes. One microgram of Luciferase Control RNA (Cat.# L4561) was added to the various RNasin® Plus:RNase A combinations, then 5µl was withdrawn and used as template for an RT-PCR reaction with the AccessQuick™ RT-PCR System (Cat.# A1701) (7) . The AccessQuick™ RT-PCR System does not contain any ribonuclease inhibitors.
As shown in Figure 1, lane 7, RNase A activity did not reappear even when heated at 70°C for 15 minutes. RNase A is a notoriously heat stable enzyme and is perfectly capable of destroying the RNA template after heating at 70°C for 15 minutes (see Figure 1, lane 6).
Heating RNasin® Plus Solutions Does Not Result In Reappearance of RNase Activity In A Complex Source of RNases
In a similar experiment to further demonstrate the range of inhibition of RNasin® Plus, a rat liver protein extract was examined as a source of ribonuclease contamination. Rat liver is known to contain at least four separate ribonucleases of the pyrimidine-specific ribonuclease superfamily (8) . Similar to the last experiment the following five solutions were prepared:
1. RNasin® Plus (40u/µl) heated at 70°C for 15 minutes.
2. RNasin® Plus (40u/µl) kept on ice.
3. Rat liver protein extract (Sigma Cat.#L-1380; resuspended at 0.5µg/µl in water) heated at 70°C for 15 minutes.
4. Rat liver protein extract (0.5µg/µl) kept on ice.
5. 20µl of RNasin® Plus and 2.5µl rat liver protein extract combined then heated at 70°C for 15 minutes.
The above solutions were assembled in various combinations, as indicated in Figure 2, and either 100ng or 10ng of the luciferase RNA was added to the reactions. Following an incubation at 37°C for 1 hour, 5µl was removed and used as a template for RT-PCR.
RNasin® Plus was shown to protect the luciferase RNA in the presence of RNase-rich rat liver extract, even with small amounts of template (Figure 2, lanes 7 and 14). The inhibition of RNases was stable when heated at 70°C for 15 minutes and inhibition continued even when the mixture was incubated at 37°C for 1 hour prior to RT-PCR.
RNasin® Plus can inhibit RNases such as RNase A and the variety of RNases found in a rat liver protein extract. The RNasin® Plus prevents these RNases from destroying an RNA template. The mixture of the RNasin® Plus and RNases can be heated to temperatures as high as 70°C for 15 minutes without reappearance of the RNase activity. Even with prolonged incubation after the heat step, no significant RNase reactivation is observed.
Related Products
Related Protocols
Related Resources
LabFact #13
When making RNase-free solutions, do not treat Tris buffers with DEPC. The DEPC reacts rapidly with amines.
Article References
- Balwit, J.M. (1996) RNasin® Ribonuclease Inhibitor: Celebrating 15 years of RNase Inhibition. Promega Notes 56, 31–3.
- Schink, M., Mei, B. and Lepinske, M. (1997) A comparison of ribonuclease inhibitors. Promega Notes 61, 30–3.
- Sambrook, J. et al. (2001) Molecular Cloning: A Laboratory Manual, 3rd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- ImProm-II™ Reverse Transcription System Technical Manual, TM236, Promega Corporation.
- Reverse Transcription System Technical Bulletin, TB099, Promega Corporation.
- Tth DNA Polymerase Product Information Sheet, 9PIM210, Promega Corporation.
- AccessQuick™ RT-PCR System Product Information Sheet, 9PIA170, Promega Corporation.
- Zhao, W. et al. (1998) Ribonucleases from rat and bovine liver: Purification, specificity and structural characterization. Biochim. Biophys. Acta 1384, 55–65.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Andrews, C., Huang, F. and Shultz, J. RNasin® Plus RNase Inhibitor: New Protein for High Temperature RNase Inhibition. [Internet] 2003. [cited: year, month, date]. Available from: https://www.promega.com/resources/pubhub/enotes/rnasin-plus-rnase-inhibitor-new-protein-for-high-temperature-rnase-inhibition/
American Medical Association, Manual of Style, 10th edition, 2007
Andrews, C., Huang, F. and Shultz, J. RNasin® Plus RNase Inhibitor: New Protein for High Temperature RNase Inhibition. Promega Corporation Web site. https://www.promega.com/resources/pubhub/enotes/rnasin-plus-rnase-inhibitor-new-protein-for-high-temperature-rnase-inhibition/ Updated 2003. Accessed Month Day, Year.
Products may be covered by pending or issued patents or may have certain limitations on use. Please visit our patent and trademark web page for more information. RNasin is a registered trademark of Promega Corporation. AccessQuick and ImProm-II are trademarks of Promega Corporation. TaqMan is a registered trademark of Roche Molecular Systems, Inc.