How Sensitivity and Crosstalk Affect Your Bioluminescent Assay Results
Doug Wieczorek, Kyle Hooper and Michael Bjerke
Promega Corporation
June 2019; tpub_212
Abstract
Success in performing bioluminescent assays is about more than just the light signal generated during the experiment. You don’t know which sample wells will give an intense signal and which ones will give a weak signal. As a result, it is inevitable that weak and strong samples will end up in neighboring wells. When this happens, how much crosstalk a plate reader allows between wells can have a significant impact on your results. You also need an instrument sensitive enough to measure weak luminescence, so that you don't miss an important "hit" or result. This article covers the topics of signal sensitivity and crosstalk in detection instruments and compares the performance of common detection instruments.
Introduction
A wide variety of applications for drug discovery require sensitive, quantitative assays. For example, studying cell signaling pathways to develop and understand new drug therapies requires assays that are able to distinguish small changes in transcription, molecular interactions and cellular health. Studying transcription can involve characterizing promoters and enhancers of transcription factors, identifying genetic point mutations or deletions, or even cellular stress causing changes in environmental conditions. Knowing how these factors affect pathway outcomes requires the ability to monitor small and subtle differences between experimental and control samples.
Sensitivity
Luminescence, fluorescence and absorbance are the three most common methods used in these assays. In general, luminescent assays are more sensitive than fluorescent assays, and fluorescent assays are more sensitive than absorbance assays. Whatever the assay method, detecting low level or small changes in a target requires a sensitive assay. For example, when studying an enzymatic reaction, you don't want to have to add a high level of substrate enzyme or catalyst to drive the reaction to a detectable level because this creates an artificial system that does not reflect physiological conditions. Instead you want your assay to be sensitive enough to be performed at near physiological levels, thus generating more biologically relevant data.
High sensitivity lets you detect samples that less sensitive methods might miss. But what happens when you have a plate of experimental samples with varying signal strength? How can you be sure that the assay signal from one well is not partially the result of a strong signal from the adjacent well? In plate readers, this is defined as well-to-well crosstalk.
Crosstalk Between Wells
Crosstalk is a function of the opacity of the plates and the masking or isolation of one well from another by the detection instrument during reading. Luminescent studies are typically performed in opaque white plates for maximum luminescent output. Unfortunately, these white plates are not completely lightproof. A strong luminescent signal in one well will contribute light to an adjacent well with low or no signal (Figure 1). This is why you should never place a positive control (high luminescence) in a well next to a negative control (low or no luminescence).
Figure 1. Schematic of well-to-well crosstalk.
A major factor in crosstalk is how well the luminescent reader isolates the signal from the well that is being measured from the signal coming from adjacent wells. Gaps between the well and the measuring device can let stray light into the detector. To circumvent this problem, you could design your experiment such that there are blank wells between your samples to avoid crosstalk from adjacent wells. However, this greatly reduces the number of samples you can measure on a microplate. If you have the option to simply use a luminometer with lower crosstalk, it's best to avoid having to design your experiment around the performance of your plate reader.
Comparing Plate Readers
Sensitive assays also require a sensitive detection platform. Plate readers that are used to detect luminescence, fluorescence and absorbance signals can vary in sensitivity (Figure 2). Factors that contribute to an instrument’s sensitivity, or limit of detection, include background noise from the detector and electronics, the type of detector that is used, and the instrument’s configuration and overall design. Lowering the instrument’s background improves the limit of detection, which in turn improves the signal-to-noise ratio, providing more usable data from each experiment. Conversely, a high background can over shadow low‐level signals, reducing the usable data from each experiment. To get a true picture of the biology in the cell, it is more relevant to measure your assay at near physiological conditions if possible (Figure 3).

Figure 2. Sensitivity of luminescent detection on GloMax® systems and other commercially available plate readers. The Bio-Glo™ Luciferase Assay System was used to test instrument sensitivity, and the limit of detection (LOD) was calculated. GloMax® Discover and Navigator had the lowest LOD at <10-19 moles luciferase.

Figure 3. Stabilizing HIF1A-HiBiT by incubation with the hypoxia mimetic 1,10-phenanthroline. HeLa cells were transiently transfected with different amounts of CMV- or PGK-driven expression constructs for HIF1A-HiBiT, diluted in carrier DNA. In parallel, HiBiT was tagged to the endogenous locus in HeLa cells using CRISPR/Cas9, and a clone was isolated. Untransfected cells, transfected cells and the endogenously tagged cell line were plated in 96-well plates and treated the following day for 4 hours with a titration of 1,10-phenanthroline. The Nano-Glo® HiBiT Lytic Reagent was added to all wells, and luminescence was measured after 10 minutes on a GloMax® Discover plate reader. Panel A. Background-subtracted luminescence shows the varying expression levels of HIF1A-HiBiT in transiently-transfected cells compared to expression from the endogenous promoter. Panel B. Data normalized to untreated cells shows how overexpression of HIF1A-HiBiT reduces the fold response from treatment with 1,10-phenanthroline. Error bars represent the standard deviation for n = 6.
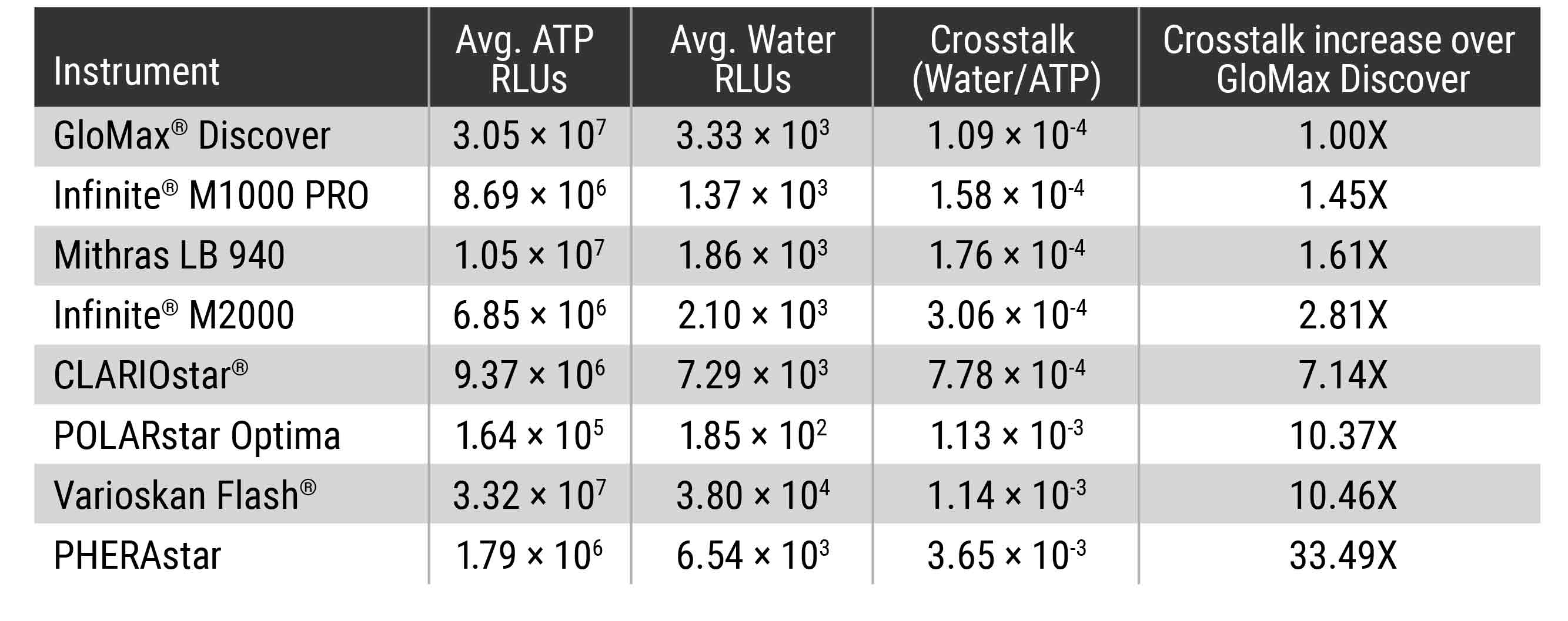
To determine what amount of crosstalk you could expect from different commonly used microplate readers, we used the Kinase-Glo® Max Reagent (Cat. #V6071) and water to assemble plates where high luminescent wells were adjacent to no sample wells. The amount of signal detected in the wells of water was compared, and the results show that the amount of crosstalk varies dramatically between instruments (Table 1).
Table 1. Detection Instrument Comparison for Crosstalk between Wells. High luminescent wells containing 100μl of a 100μM ATP solution plus 100μl of Kinase-Glo® Max Reagent were plated on a standard 96-well opaque white plate next to water-only wells (200µl Nuclease-Free Water). Signal in the water-only wells was measured after room temperature incubation for 10 min to achieve steady-state luminescence (luminescent half-life ~5 hours). A blank plate containing only 200μl of water/well was prepared to determine background. The background and experimental plates were read in the different microplate readers with 0.5sec integration times per well. The average background was determined for each instrument and this average was subtracted from the water-only and ATP/Kinase-Glo® Max wells. The average water-only RLUs were determined and the average ATP/Kinase-Glo® Max RLUs determined. The average water-only RLUs was divided by the average ATP/Kinase-Glo® Max RLUs to calculate the crosstalk value.
Conclusion
It is easy to focus on the sensitivity of your assay and forget that an assay’s results are only as sensitive as the detection level of the instrument. The combination of instrument and assay sensitivity provide the true level of detection for your experiment. Having an instrument with the sensitivity necessary to achieve the level of detection you need means you will need to use less sample, less enzyme and less catalyst to perform your experiments. You will also be able to detect fewer cells, lower levels of transcription and more subtle changes in molecular interactions. Sensitivity comparisons (LOD and LOQ) between commercially available detection instruments indicated GloMax® Discover and Navigator exhibited 1 to 2 logs better sensitivity of the instruments tested (Figure 2). The low instrument background and overall design means that the results detected for each well by the GloMax® instruments give you best chance to measure near physiological levels in luminescent assays.
Crosstalk comparisons between commercially available detection instruments indicated GloMax® Discover exhibited the least amount of crosstalk of the instruments tested. The proprietary masking design of the instrument, which isolates signal of the desired well from that of a neighboring well, means that the results detected for each well by the GloMax® microplate readers can be trusted without worrying about the signal in adjacent wells.
Faster, Easier Data Analysis
From advanced multimode luminescence, fluorescence, absorbance, BRET and FRET capabilities to dedicated microplate or single-tube luminometers, there is a GloMax® system for every lab and throughput need.