Adeno-Associated Virus Research
Adeno-associated virus (AAV) has become one of the most promising viral vectors for gene therapy. Recombinant AAVs are efficient at delivering genetic materials to both dividing and non-dividing cells. AAVs are replication defective, non-pathogenic, non-cytotoxic and can maintain physiologically relevant expression levels in cells and tissues. Many challenges exist for developing AAV-based gene therapy including lack of simple standardized analytical methods, challenges for manufacturing scalability and the presence of AAV neutralizing antibodies in most human populations.
To help with some of these challenges, we offer a growing portfolio of novel AAV solutions based on our bioluminescent technology, proteomics and genomics tools.
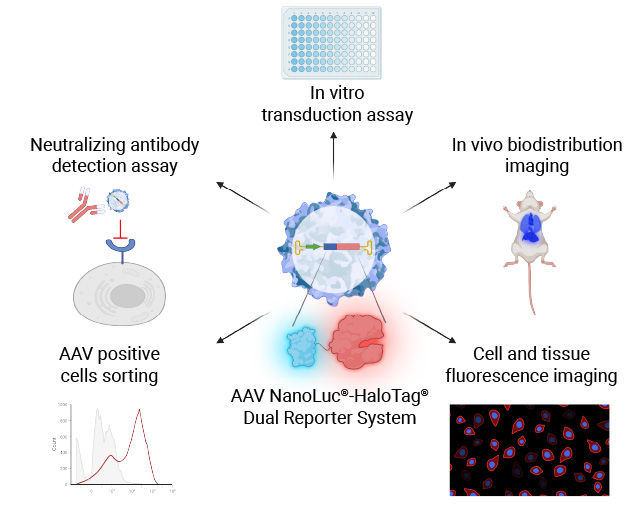
NanoLuc®-HaloTag® Dual Reporter AAV System
The sensitive and versatile AAV NanoLuc®-HaloTag® Dual-Reporter System can be used for a variety of applications including novel serotype screening, determining in vivo biodistribution and neutralizing antibody detection.
Learn more about NanoLuc® luciferase and HaloTag® technology.
Contact us for information on purchasing the AAV dual reporter systems.
Measure In Vitro Cell Transduction (Infectivity)
Transducing cell lines with AAV NanoLuc®-HaloTag® reporter for each AAV serotype allows you to measure the selectivity of each AAV serotype for the cell line transduced.
If transduction is successful, the expressed NanoLuc® luciferase will produce a luminescent signal in the presence of a substrate. The resulting luminescent signal intensity is proportional to the transduction efficiency of AAV.
NanoLuc® Luciferase Assay Workflow
The simplicity of the NanoLuc® Luciferase Assay workflow enables rapid, sensitive testing of cell tropism by AAV serotype.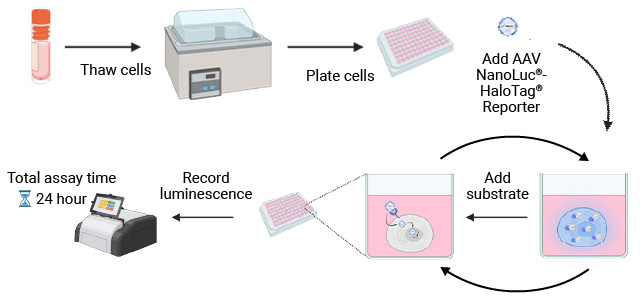
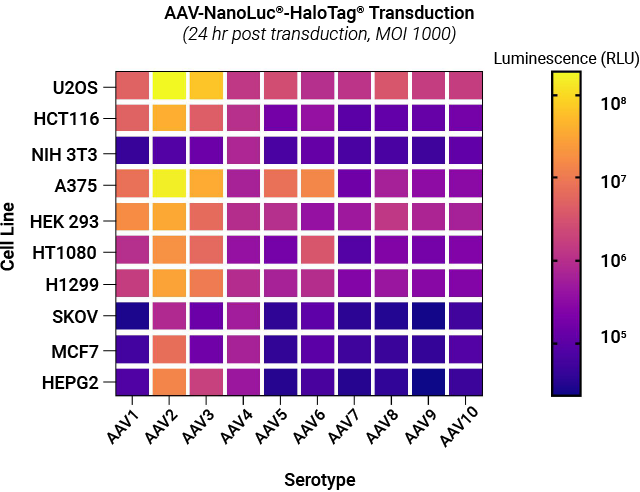
Selectivity of AAV NanoLuc®-HaloTag® reporters toward different cell lines. A panel of AAV NanoLuc®-HaloTag® reporters for AAV serotypes 1–10 were examined against a panel of cell lines representing different tissue types. Each cell type was transduced with reporters for each AAV serotype at MOI of 1,000. Following substrate addition, the luminescent signal produced by the infected cells expressing NanoLuc® luciferase was measured. The signal intensity is proportional to the transduction efficiency of AAV.
+ Janelia Fluor® HaloTag® 646 Ligand
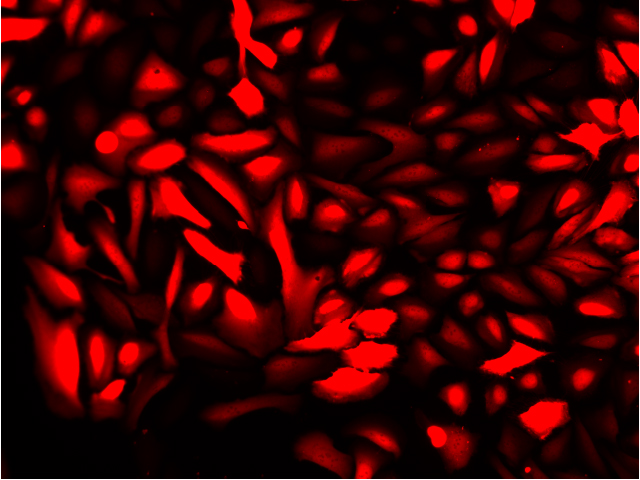
Overlay with DNA Stain (Blue)
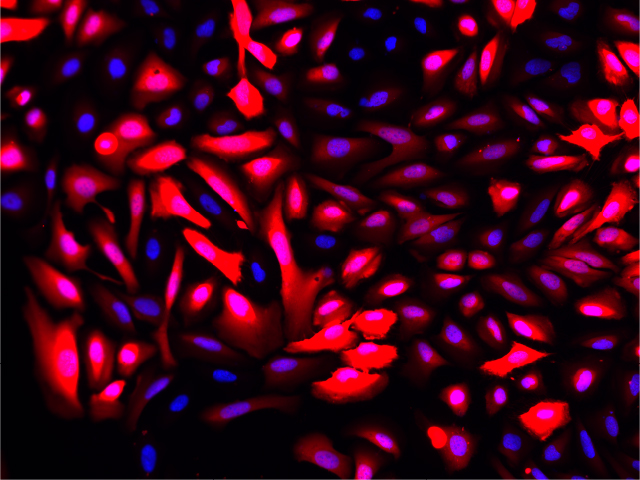
Fluorescence imaging of AAV infected cells. U2OS cells were transduced with an AAV2-NanoLuc®-HaloTag® reporter. Cells were incubated with Janelia Fluor® HaloTag® 646 Ligand 48 hours post-transduction. Images were taken using a Keyence microscope. Cells expressing HaloTag® proteins are in red and DNA stain are in blue.
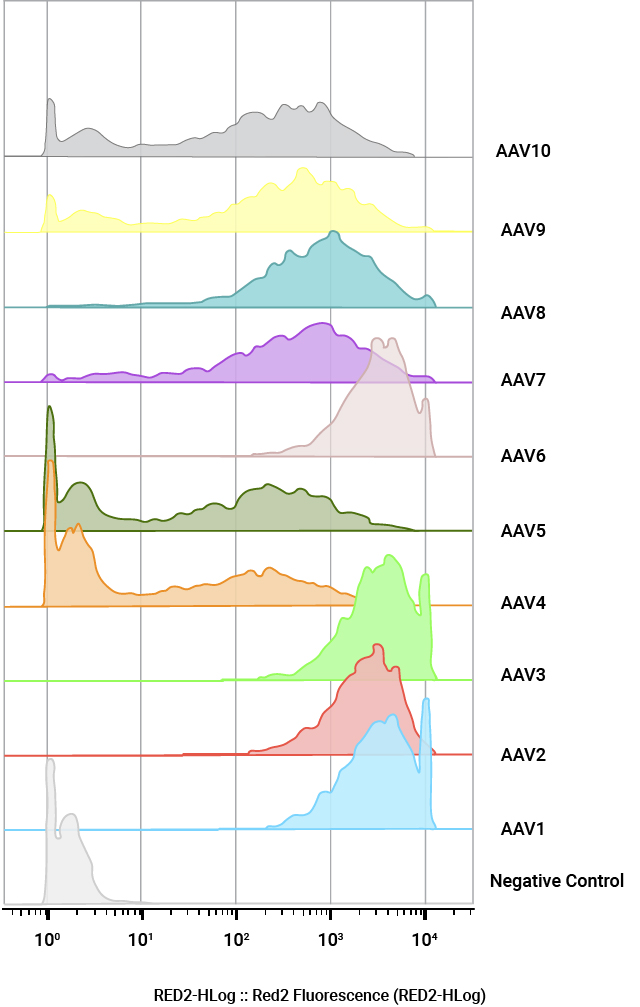
Quantification of AAV positive cells by flow cytometry. U2OS cells were infected with a panel of AAV NanoLuc®-HaloTag® reporters (AAV1–AAV10) at MOI of 100,000. Cells were labeled with Janelia Fluor® HaloTag® 646 Ligand 48 hours post-transduction and quantified by flow cytometry.
Detecting In Vivo Tropism and Biodistribution with NanoLuc® Luciferase
NanoLuc® luciferase and the Nano-Glo® Fluorofurimazine In Vivo Substrate (FFz) offer a new reporter option for AAV in vivo biodistribution studies. Optimized specifically for in vivo detection of NanoLuc® luciferase, the Nano-Glo® FFz Substrate is an aqueous-soluble detection reagent with increased substrate bioavailability that leads to bright, stable signals. Nano-Glo® FFz has handling requirements compatible with in vivo workflows, offers flexible delivery options and the substrate specificity allows NanoLuc® and firefly luciferases to be used together for dual-luciferase imaging studies.
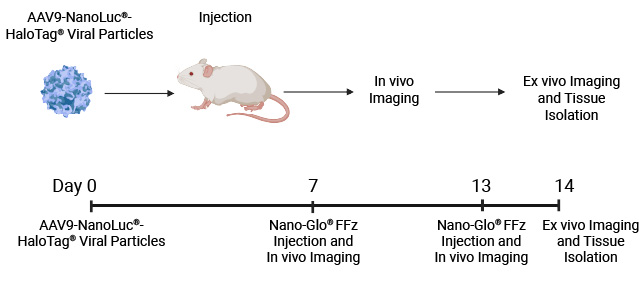
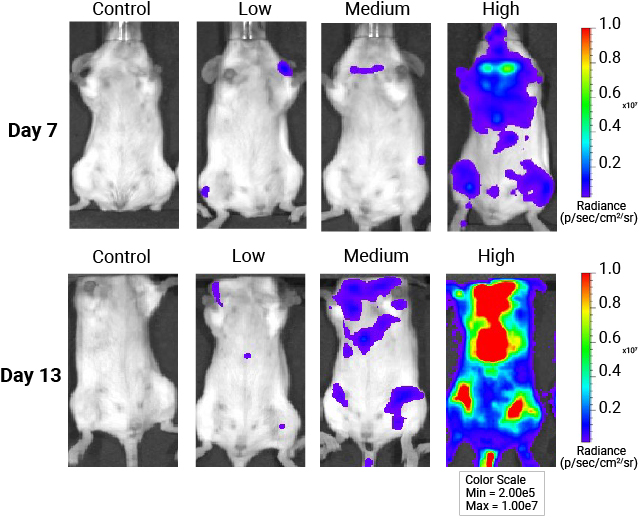
In vivo whole-body imaging of mice injected with AAV9-NanoLuc®-HaloTag® viral particles. Mice were injected with increasing levels of AAV9-NanoLuc®-HaloTag® viral particles. Nano-Glo® FFz (0.44μmoles) was injected 7- and 13-days post-transduction followed by in vivo imaging. Tissue tropism starts to appear at 7 days, and a stronger signal and more extensive tissue tropism is visible after 13 days. Bioluminescence imaging was done at the University of Wisconsin Small Animal Imaging and Radiotherapy Facility.
AAV Viral Genome Analysis Workflow for In Vivo Biodistribution Studies
Our DNA extraction and quantification solutions enable you to detect and quantify AAV viral genome in various tissues using a fast (~5 hours), high-throughput workflow.

To further simplify the workflow, we have developed an analysis software to automate custom data analysis and visualization. Download this flyer to learn more.
No Viral DNA Detected in Brain

ddPCR results quantifying AAV copies in brain tissue. Mice were injected with varying levels of AAV9-NanoLuc®-HaloTag® viral particles. DNA was extracted from tissues collected 14 days post-injection. ddPCR amplification was performed using ITR and TERT (house-keeping gene control) primers and probes.
AAV Viral DNA Primarily Accumulated in Liver

ddPCR results quantifying AAV copies in liver tissue. Mice were injected with varying levels of AAV9-NanoLuc®-HaloTag® viral particles. DNA was extracted from tissues collected 14 days post-injection. ddPCR amplification was performed using ITR and TERT (house-keeping gene control) primers and probes.
Determining NAb Titer in Human Serum

Determination of NAb titer in human serum samples. Serum samples were serially diluted to determine NAb titer, which is defined as the lowest dilution factor that reaches 50% transduction efficiency. Transduction efficiency of the AAV-NanoLuc® viral particles was measured using luminescent detection of NanoLuc® luciferase expression.

Specificity of NanoLuc® luciferase-based NAb assay. Removing the antibodies from seropositive samples recovers transduction efficiency of the AAV-NanoLuc® viral particles as measured by NanoLuc® luciferase expression. The recovery suggests the specificity of the NanoLuc® luciferase-based NAb assay.
Capsid Titer
The Lumit® Immunoassay technology provides a faster, more reliable alternative to traditional ELISAs, eliminating variability associated with multiple wash steps and offering the convenience of simple, sensitive luminescent detection.
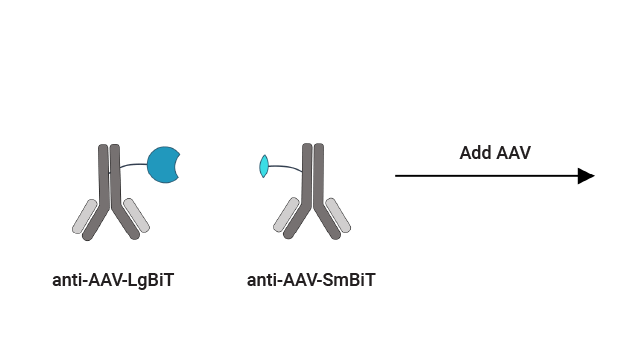
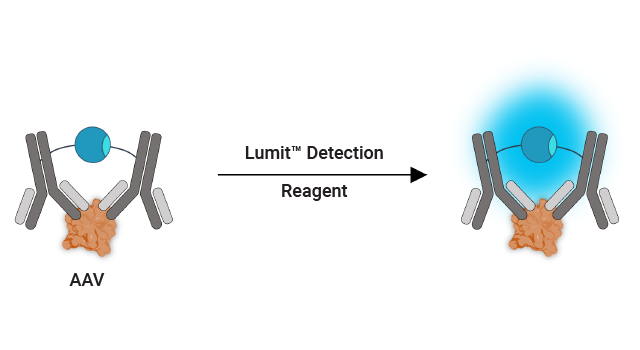
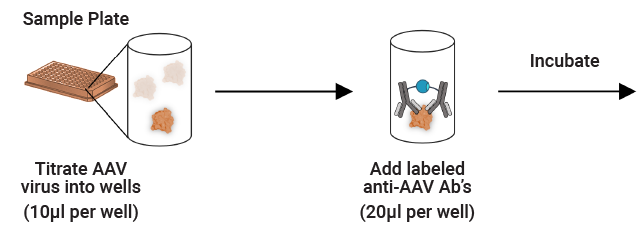
Immunoassay for AAV Capsid Titer
The Lumit® AAV Capsid Immunoassay can be used to quantify intact capsids over a broader dynamic range than traditional ELISAs.
The assays can be used with purified samples or in-process crude lysates. The simple workflow can be implemented throughout the manufacturing process to optimize transfection, purification and assess AAV yields.
Lumit® AAV Capsid Immunoassay Workflow

Lumit® Immunoassays quantify intact AAV capsids with a dynamic range of 3 orders of magnitude. Lumit® Immunoassays were performed on purified capsids using two distinct anti-AAV9 antibodies and results were compared to those of a commercially available anti-AAV9 ELISA assay.

Lumit® Immunoassays show a broad linear range for AAV capsids. Lumit® Immunoassays were performed on purified capsids using anti-AAV2, anti-AAV6 and anti-AAV8 antibodies.
Quantifying AAV Genome Titer
DNase is commonly used prior to qPCR or dPCR to determine AAV genome titer. Inconsistent digestion by DNase treatment can result in variable qPCR or dPCR data. Using the TruTiter™ Reagent Kit, only DNA inside an intact capsid is amplified. This enables true quantification of AAV viral genome and eliminates the need for a DNase treatment step.
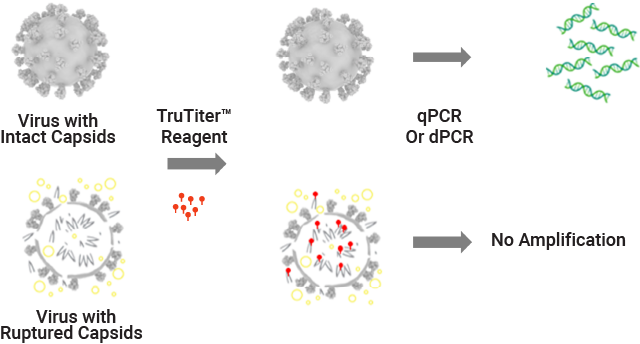
With TruTiter™ Reagent, only the DNA inside the intact capsid can be amplified for accurate genome titer.
TruTiter™ Reagent Kit Allows a Fast and Simple Assay Workflow
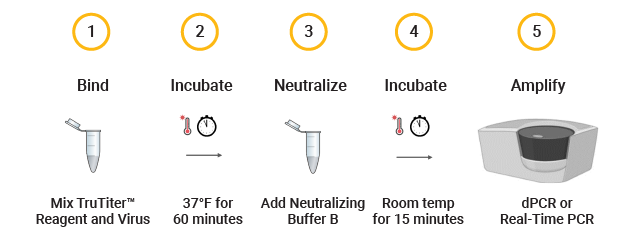
TruTiter™ Reagent Results in Less Variability
Compared to DNase-Based dPCR Workflows

AAV2-NanoLuc® viral capsids (1010GU/ml) were treated at 65°C for 10 minutes. Following heat treatment, the AAV particles were treated with either DNase (red) or TruTiter™ Reagent (green) and amplified using dPCR with 8 technical replicates each.

AAV2-NanoLuc® viral capsids (1010GU/ml) were treated with 0.1% Tween®-20 for 10 minutes. Following detergent treatment, the AAV particles were treated with DNase (red) or TruTiter™ Reagent (green) and amplified using dPCR, with 8 technical replicates each.

AAV2-NanoLuc® viral particles (107GU/ml) were treated at the indicated temperature for 10 minutes. Following temperature treatment, the AAV particles were treated with DNase (red) or TruTiter™ Reagent (green) followed by dPCR. In parallel, a 24-hour infectivity assay (blue) was performed using HEK293 cells. To aid interpretation, data is represented as percent of signal achieved with the room temperature (R.T.) treatment.
Peptide Mapping Reagents for LC-MS Analysis of AAV Capsid Proteins
The magnetic SP3 beads offer efficient contamination removal, can denature the substrate under non-denaturing proteolysis conditions and can help concentrate dilute samples for LC-MS/MS analysis.
Interested in alpha testing the SP3 beads? Contact us.
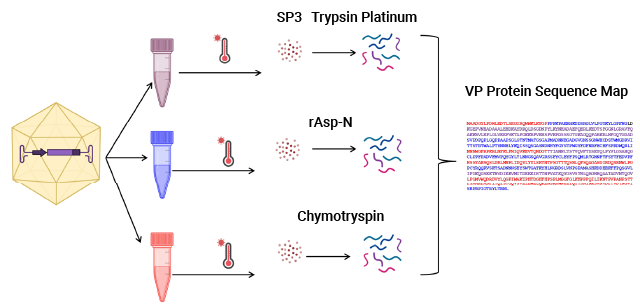
The SP3 Workflow for Capsid Proteolysis
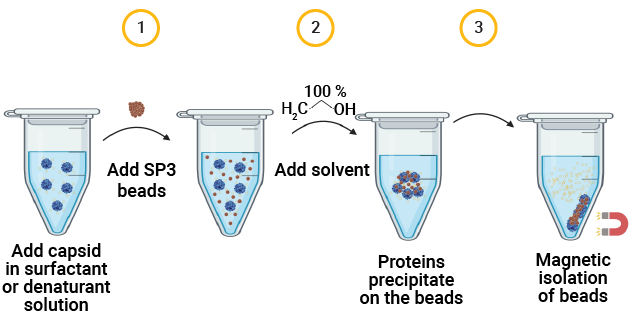
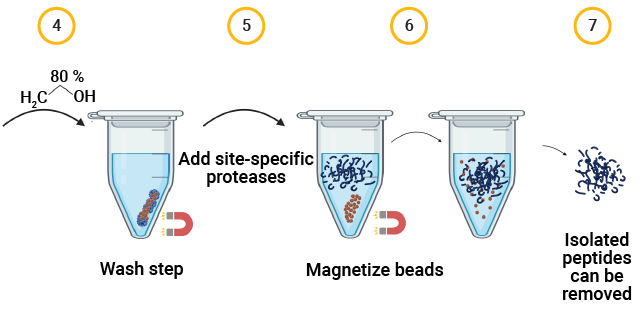
AAV capsid protein peptide mapping sequence coverage. Capsid protein was processed using the magnetic SP3 beads. Proteolysis was performed at low pH in AccuMAP™ 10X Low pH Reaction Buffer using Trypsin Platinum, Arg-C Ultra, rAsp-N and Chymotrypsin. E/S ratios were 1:25 and proteolysis was allowed to proceed for 18 hours at 37°C. Peptides were removed from the SP3 beads and desalted (C18) prior to LC-MS/MS on a Thermo Scientific Orbitrap Exploris™ 240 Mass Spectrometer interfaced with a Thermo NanoLC 1200 LC System.
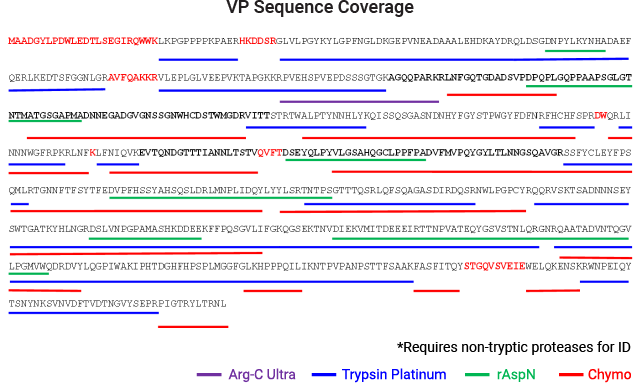
Peptide Mapping Reagents for LC-MS Analysis of AAV Capsid Proteins
| Reagent |
Primary Utility |
Additional Information |
|---|---|---|
| Trypsin Platinum |
Capsid protein sequence mapping |
Recombinant Trypsin with very high cleavage specificity and stability |
| Arg-C Ultra |
Additional capsid protein coverage |
Please inquire |
| Chymotrypsin | Additional capsid protein coverage |
Needed to map acidic regions of capsid not covered by Trypsin |
| rAsp-N | Additional capsid protein coverage |
Needed to map regions of capsid not covered by Trypsin |
| SP3 Sample Cleanup Kit |
Key sample prep tool for protein capture and digestion |
Compatible with all surfactants and effective for enrichment of dilute protein (virus) solutions. Please inquire |
| Rapid Trypsin/Lys-C |
Effective tool for mapping deamidation sites |
|
| PNGase F |
Critical for mapping glycosylation sites |
|
| AccuMAP® 10X Low pH Reaction Buffer |
Minimization of capsid protein deamidation |
Required for proteolysis prior to successful LC-MS/MS and peptide mapping analysis |