The Fundamentals of ADCs: Which Assay is Right for Your Work?
Kai Hillman, PhD, Promega Corporation
Publication date: September 2025
What are ADCs and How Do They Work?
Antibody–drug conjugates (ADCs) represent a promising frontier in targeted cancer therapy, marrying the specificity of monoclonal antibodies with the potent cell-killing capabilities of cytotoxic drugs. As complex bioconjugates, ADCs use multifaceted mechanisms of action (MOA) to deliver their therapeutic payloads precisely to tumor cells. In recent years, the clinical landscape has witnessed accelerated development and approval of ADCs, reflecting both their innovative design and the expanding understanding of their molecular dynamics. Approved ADCs deploy a variety of MOAs—including direct cytotoxicity, bystander killing, and receptor blockade—while also engaging immune-mediated processes such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) (1). Looking ahead, next-generation payloads aim to broaden this therapeutic arsenal by incorporating immune modulation, altering protein expression, and directly targeting specific oncogenic mutations.
At its core, an ADC is composed of three critical components: the antibody, the linker, and the payload. The antibody confers target specificity, binding with high affinity to tumor-associated antigens, thereby ensuring that the cytotoxic drug is delivered selectively to cancer cells. The linker, which bridges the antibody and the payload, is engineered to be stable in circulation yet labile enough to release the drug once internalized by the target cell. This balance in linker design is pivotal for optimizing therapeutic efficacy while minimizing off-target toxicity. Finally, the payload itself is typically a highly potent cytotoxic agent, capable of inducing cell death even at very low concentrations, a necessity given the limited number of molecules delivered per antibody (Figure 1).
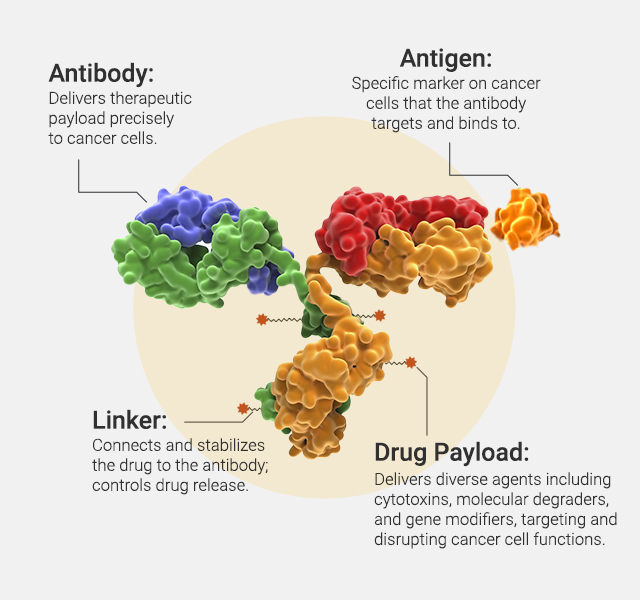
How Do ADCs Modulate Immune Response?
Beyond acting as delivery vehicles, the antibodies in ADCs play an active role in modulating immune responses through their Fc regions. The Fc domain can engage immune effector cells by binding to Fc receptors on natural killer cells, macrophages, and other components of the immune system. The majority of ADCs use IgG1 antibodies, due to their long serum half-life and robust Fc effector functions. However, some ADCs leverage differing affinities of IgG isotypes for FcyR to help shape the immune response. Using immunoassay approaches, such as the Lumit® FcγR Binding Immunoassays, researchers can quickly measure antibody and receptor interactions. Fc effector functions can include ADCC, ADCP, and CDC, thereby contributing an additional layer of antitumor activity.
The immune-mediated functions not only assist in the direct killing of tumor cells but also help in shaping the tumor microenvironment to favor an immune response against cancer. As such, modifications in the Fc region are being explored to fine-tune these interactions and enhance overall therapeutic outcomes (2). Just like unconjugated monoclonal antibody drugs, regulators will likely require the Fc function of ADCs to be evaluated. Published studies of some drugs have shown that payload conjugation has little impact on Fc function (3), but as conjugation chemistry evolves, sometimes enabling very large drug-antibody-ratios (DAR), it is essential to quantify to what extent Fc function has been preserved in the final drug substance. Functional reporter assays, such as the ADCC and ADCP Reporter Bioassays can provide ADC developers the measurements required for ranking their effector functions.
How Can You Measure ADC Internalization?
How Can You Determine the Cytotoxicity of the ADC Payload?
What Is ADC Bystander Killing Effect and How Is It Measured?
Summary
Citations
- Crescioli, S. et al. (2025) Antibodies to watch in 2025. MAbs 17, 2443538.
- Hoffmann, R.M. et al. (2018) Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). Oncoimmunology 7, e1395127.
- Junttila, T.T. et al. (2011) Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 128, 347–356.
- Du, X. et al. (2008) Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 68, 6300–6305.
- Kovtun, Y.V. et al. (2006) Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 66, 3214–3221.
- Hurvitz, S.A. et al. (2023) Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117.
Learn More
Related Resources
Antibody-Drug Conjugate (ADC) Discovery and Development
Enhance your ADC research with our suite of technologies tailored to support every phase of development.
Bioassay Qualification
We offer bioassay qualification services to facilitate downstream validation studies in your laboratory or with a CRO partner