Direct Amplification from Buccal and Blood Samples Preserved on Cards Using the PowerPlex® 16 HS System
Promega Corporation
Publication Date: 2009
Introduction
Short tandem repeat (STR) analysis is a technique commonly used by forensic and paternity laboratories for genetic identification. For known-sample testing, such as paternity, reference or convicted offender typing, blood and buccal samples often are used. Samples often are collected on special paper cards, such as FTA® and Protein Saver® 903 paper, which contain chemicals that lyse cells and preserve DNA for future analysis. These cards can be stored long-term at room temperature, providing a simple, stable and inexpensive means of storing DNA samples.
Blood and buccal samples often contain substances that can inhibit DNA amplification. In the past, DNA stored on paper cards was often extracted from the cards and further purified to remove these inhibitors. The ability to directly amplify preserved DNA would allow the user to process and analyze DNA samples much more quickly and efficiently. By eliminating the need for DNA purification, the user saves reagent costs and the time required for purification. Forensic databasing labs and paternity labs with high volumes of samples to analyze would clearly benefit from an effective and consistent method of direct amplification.
Promega recently introduced the PowerPlex® 16 HS System (Cat.# DC2101), which allows co-amplification of 16 STR loci. The PowerPlex® 16 HS System uses a hot-start Taq DNA polymerase and is more tolerant of PCR inhibitors than competing STR systems, including the original PowerPlex® 16 System, making it ideal for use in direct amplification. Here we demonstrate the effectiveness of the PowerPlex® 16 HS System for direct amplification of DNA from buccal and blood samples preserved on FTA® and Protein Saver® 903 cards.
“We demonstrate the effectiveness of direct amplification of DNA from buccal and blood samples preserved on FTA® and Protein Saver® 903 cards using the PowerPlex® 16 HS System.”
Materials and Methods
Buccal samples from 11 donors were collected using the EasiCollect™ sample collection device (Whatman, Kent, UK) following the manufacturer’s instructions. Blood samples from 11 donors were collected and preserved in EDTA-anticoagulated Vacutainer® Blood Collection Tubes (Becton Dickinson, Franklin Lakes, NJ) and applied to FTA® cards within the Sampact™ Collection Device (Fitzco, Spring Park, MN) as well as Protein Saver® 903 cards (Whatman). All samples were allowed to dry completely prior to processing.
We tested a 0.5–2.0mm range of punch sizes (data not shown) and chose to use 1.2mm punches. Punches were taken from each card in quadruplicate using the Harris 1.2mm Micro Punch and added to a 96-well plate for direct amplification. The PowerPlex® 16 HS System was used to amplify STR regions from all preserved DNA samples. A 25µl reaction mixture consisting of 5µl of 5X Master Mix, 2.5µl of 10X Primer Pair Mix and 17.5µl of Water, Amplification Grade, was added to all punch samples in the 96-well plate.
Amplification was performed with a GeneAmp® PCR System 9700 thermal cycler using the protocol recommended in the PowerPlex® 16 HS System Technical Manual #TMD022. We selected 28 cycles (10/18) of amplification for the 1.2mm punches based on initial testing of 26–32 cycles (data not shown). Following amplification, 1µl of each reaction was analyzed using an ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems) with a 3kV, 5-second injection to detect amplified fragments. Data analysis was performed using the GeneMapper® ID software, version 3.2. Analysis conditions included peak amplitude thresholds set at 50RFU with 20% peak filtering for tetra- and pentanucleotide marker repeats as recommended in the PowerPlex® 16 HS System Technical Manual.
Results
EasiCollect™ Device—Buccal Samples
A key aspect of direct amplification is the ability to provide consistent and reproducible results to generate the correct genetic profiles. Of the 44 buccal sample punches from EasiCollect™ collection devices, 43 resulted in full genetic profiles. Only one sample punch failed to give a full profile, exhibiting dropout of six of the possible 31 alleles. It should be noted that three other punches from the same donor card resulted in full profiles. Overall, EasiCollect™ punches with buccal samples resulted in a full-profile success rate of 98%. Heterozygous peak heights for all 44 samples averaged 2709 ± 1659RFU (Table 1). The majority of heterozygous peaks heights were in the range of 1000–4000RFU. Average peak heights from punches from the same donor were consistent. EasiCollect™ devices use an indicating dye to indicate transfer of the buccal sample to the card; however, even cards that appeared to show relatively little sample transfer yielded good results. Locus-to-locus balance also was good for EasiCollect™ samples (data not shown). Figure 1, Panel A, shows an electropherogram of a representative profile from an EasiCollect™ buccal sample.
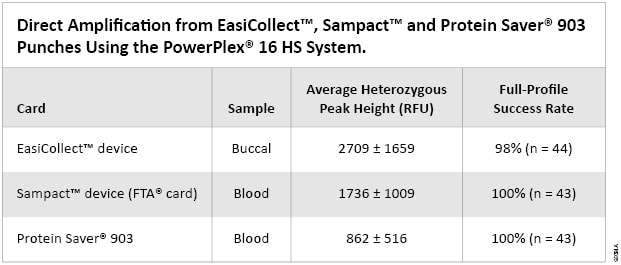 Table 1. Direct Amplification from EasiCollect™, Sampact™ and Protein Saver® 903 Punches Using the PowerPlex® 16 HS System.
Table 1. Direct Amplification from EasiCollect™, Sampact™ and Protein Saver® 903 Punches Using the PowerPlex® 16 HS System. Sampact™ Device (FTA® Card)—Blood Samples
Of the 44 blood sample punches from Sampact™ collection devices (FTA® card), 43 resulted in full genetic profiles. Analysis of one sample punch yielded a failed internal lane standard (ILS), and the sample could not be analyzed. Thus, full profiles were obtained with 43 of 43 samples (100%) that were analyzed. Heterozygous peak heights for all 43 samples averaged 1736 ± 1009RFU (Table 1). The majority of heterozygous peaks heights were in the range of 1000–2500RFU. Average peak heights from punches from the same donor were consistent. Figure 1, Panel B, shows an electropherogram of a representative profile from a Sampact™ (FTA® card) blood sample.
Protein Saver® 903 Cards—Blood Samples
Of the 44 blood sample punches from Protein Saver® 903 cards, 43 resulted in full genetic profiles. One sample punch could not be analyzed due to a poor injection. Thus, full profiles were obtained with 43 of 43 samples (100%) that were analyzed. Heterozygous peak heights for all 43 samples averaged 862 ± 516RFU (Table 1). The majority of heterozygous peaks heights were in the range of 500–1500RFU. Average peak heights from punches from the same donor were consistent. Figure 1, Panel C, shows an electropherogram of a representative profile from a Protein Saver® 903 blood sample.
 Figure 1. Representative electropherograms for direct amplification of DNA from three different donor buccal and blood samples preserved on various card types.
Figure 1. Representative electropherograms for direct amplification of DNA from three different donor buccal and blood samples preserved on various card types.
Panel A. EasiCollect™ device with buccal sample. Panel B. Sampact™ device (FTA® card) with blood sample. Panel C. Protein Saver® 903 card with blood sample.
Considerations for Successful Direct Amplification
In the past, direct amplification of preserved DNA from storage cards was problematic due to the presence of inhibitors that can lead to allele dropout. The PowerPlex® 16 HS System was recently introduced in response to the growing need for an STR system capable of generating full genetic profiles with all samples, including forensic casework samples known to contain a variety of impurities that inhibit amplification. The PowerPlex® 16 HS System is more tolerant of PCR inhibitors than competing STR systems, generating profiles from samples that previously failed to amplify(1)(2)(3). These qualities enable direct amplification as a valid and effective application of this STR system, allowing the user the flexibility to use the same STR system for both casework and direct amplification protocols and eliminating the need for a second kit.
A number of parameters need to be considered for successful direct amplification, including sample type, collection card type, punch diameter, number of amplification cycles and capillary electrophoresis injection conditions. Our initial screen of blood and buccal samples on the various card types included punch sizes ranging from 0.5mm to 2.0mm, with amplification conditions ranging from 26 to 32 cycles. Many potential combinations of punch sizes and cycle numbers exist and allow the user to tailor parameters based on card and sample types. Our studies using 1.2mm punches and 28 cycles indicate that these conditions may be amenable to most cards and sample types tested (data not shown). Although using smaller punch sizes resulted in less overall card use per punch, the two smallest punch sizes tested, 0.5mm and 0.75mm, were difficult to work with due to the static effects of the cards. Furthermore, the 1.2mm punch size is compatible with punch robots such as those from BSD Robotics (Australia), making the process adaptable to automation.
Although our results suggest that the amplification parameters used in this study will work for a broad range of sample and card types, each laboratory should perform their own in-house optimization experiments. For example, a slight increase or decrease in cycle number or punch size should be considered. In addition, variation in instrument sensitivity and laboratory preferences for signal strength necessitates lab-specific optimization of injections conditions. Preliminary results with other card types were highly variable in average peak heights and locus-to-locus balance. In many of these cases, further optimization would likely produce more consistent peak heights in the proper range. However, some other collection devices and techniques have inherent variation in sample deposition that may prove difficult to overcome with STR assay design.
Conclusions
We have demonstrated the effectiveness of using the PowerPlex® 16 HS System for direct amplification of DNA from buccal and blood samples preserved on cards for STR analysis. The time- and cost-saving benefits associated with this application can be a major advantage to database and paternity laboratories where backlogs of unanalyzed samples may be high and funds for processing may be tight. Additionally, this study demonstrates that a single kit commonly used for casework also can be used effectively for direct amplification in database and paternity applications. This increases the consistency of product use across large laboratory systems.
Related Products
Related Articles
Article References
- Ensenberger, M.G. and Fulmer, P.M. (2009) The PowerPlex® 16 HS System. Profiles in DNA 12(1), 9–11.
- McLaren, R. (2007) PowerPlex® 16 versus Identifiler® Systems—Sensitivity and effects of inhibitors. #AN156
- (2007) AmpFlSTR® MiniFiler™ PCR Amplification Kit User Guide, Applied Biosystems.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Wieczorek, D. and Krenke, B. Direct Amplification from Buccal and Blood Samples Preserved on Cards Using the PowerPlex® 16 HS System. [Internet] 2009. [cited: year, month, date]. Available from: https://www.promega.com/resources/profiles-in-dna/2009/direct-amplification-from-buccal-and-blood-samples-preserved-on-cards-using-powerplex-16-hs/
American Medical Association, Manual of Style, 10th edition, 2007
Wieczorek, D. and Krenke, B. Direct Amplification from Buccal and Blood Samples Preserved on Cards Using the PowerPlex® 16 HS System. Promega Corporation Web site. https://www.promega.com/resources/profiles-in-dna/2009/direct-amplification-from-buccal-and-blood-samples-preserved-on-cards-using-powerplex-16-hs/ Updated 2009. Accessed Month Day, Year.
Contribution of an article to Profiles in DNA does not constitute an endorsement of Promega products.
Products may be covered by pending or issued patents or may have certain limitations. More information.
All prices and specifications are subject to change without prior notice.
Product claims are subject to change. Please contact Promega Technical Services or access the Promega online catalog for the most up-to-date information on Promega products.