Detecting Ozone-Induced Changes in Cellular Redox Balance via GSH/GSSG-Glo™ Assay
Biology Department, Davidson College, Davidson, North Carolina 28035
Publication Date: 2011
Abstract
Ozone exposure causes pulmonary damage by a number of mechanisms that may result in the observable deleterious health effects seen in urban areas with high ozone levels. We are interested in understanding the mechanisms by which lung cells respond to and mediate ozone-induced damage. Here we demonstrate the flexibility of the Promega GSH/GSSG-Glo™ Assay to evaluate the redox state of cells in experiments where exposure to different levels of the test compound cannot occur in the same 96-well plate.
Introduction
Epidemiological studies reveal an association between lung disease and ozone exposure. Steadily increasing levels of ambient ozone in urban areas correlate with increasing incidences of acute respiratory distress syndrome (ARDS), asthma and lung cancers, and patients with these diseases experience more serious symptoms on days with higher ambient ozone levels(1)(2)(3). Additional studies have shown that ozone exposure induces acute and chronic pulmonary inflammation, both of which are postulated to be part of the pathogenic process of chronic obstructive pulmonary diseases (COPD), ARDS, asthma and idiopathic pulmonary fibrosis (IPF)(4). The effect of environmental stressors, like ozone, on physiological responses has been outlined in whole organism studies. Ozone exposure in the lungs can cause lipid and protein peroxidation in the epithelial lining fluid and on the cell surface, increase reactive oxidative species (ROS) in the cell and induce DNA damage and cell death(1)(5)(6)(7). In addition, whole organism studies document increased production of glutathione post-ozone exposure. Upregulated expression of this antioxidant is hypothesized to minimize the harmful effects of the strong oxidant and to restore redox homeostasis(8)(9)(10). Consequently, the effect of ozonation on cellular health can be deduced by monitoring total glutathione and the ratio of glutathione in reduced (GSH) and oxidized (GSSG) forms. Whole animal studies have strengths in that the experimental system contains the variety of interactions seen in the body. However, these models can confound analysis due to the interaction of oxidants with nontarget cell types. Cell culture studies allow interactions and reactions specific to critical target cell types to be investigated more thoroughly. Type II alveolar cells are the site of gas exchange and integral to lung function, but their responses to ozonation have not been fully characterized. To model the effects of environmental oxidants on these cells in culture, we examined the effect of ozone on redox homeostasis in rat type II pneumocytes (L2 cells).
Results
L2 cells were seeded at 104 cells/well, exposed for 1 hour to 3.5L/minute CO2 + 350ppb O3 (hazardous level of O3 as defined by the EPA) then allowed to recover for 24 hours. As a measure of cellular health and the redox state of the cells, total glutathione and GSSG levels were determined using the GSH/GSSG-Glo™ Assay (Cat.# V6611). Changes in the levels of total glutathione detected are consistent with previous whole animal studies, suggesting that alveolar cells use glutathione to mitigate the effect of ozone exposure(11). Our data differ from the whole animal reports in that a) only a single cell type is being analyzed under controlled conditions, b) pneumocytes, not bronchial or tracheal cells, are the focus and c) ozone exposure is within environmentally relevant levels. We found that L2 cells exposed to 350ppb O3 had a small but statistically significant increase in total glutathione (Figure 1, Panel A; p<0.0001). Further analyses show that O3 exposure resulted in a twofold increase in the concentration of oxidized glutathione (Figure 1, Panel B) such that the ratio of total to oxidized glutathione decreased by 50% in the presence of 350ppb ozone (Figure 1, Panel C).
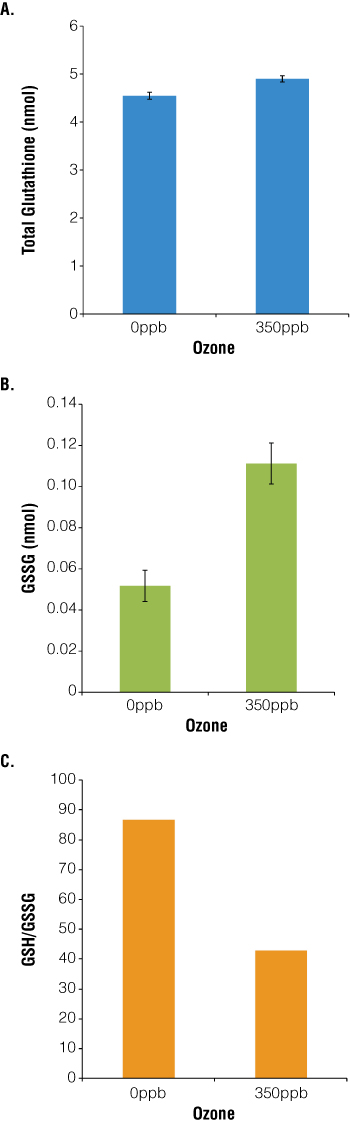 Figure 1. O3 exposure increases absolute and relative levels of glutathione and GSSG.
Figure 1. O3 exposure increases absolute and relative levels of glutathione and GSSG.
104 cells were seeded in DMEM + 10% FBS in white-walled 96-well plates (Costar) and allowed to attach for 48 hours at 5% CO2/air, 37°C. Ozone was generated from O2 via Ozone Gas Generator (Pacific Ozone Technology) and diluted with 5% CO2/air. Before exposure, medium was exchanged for HBSS, and cells were exposed to 37°C, 3.5L/minute 5% CO2/air + 350ppb O3 for 1 hour, then allowed to recover for 24 hours in fresh medium. Total glutathione (Panel A), GSSG levels (Panel B) and the oxidative ratio (total glutathione/GSSG; Panel C) were determined using the GSH/GSSG-Glo™ Assay with luminescence measured by the FLx800™ Multidetection Microplate Reader (BioTek). Graphs represent the mean ± S.E.M, n = 30 (Panel A: p<0.0001; Panel B: p<0.0001).
A priori, we developed two hypotheses to describe alveolar cells’ potential responses to oxidative stress. In the first, ozone exposure causes an increase in cellular antioxidants, and thus the total amount of glutathione increases. Because there is usually 100 times more GSH than GSSG in the cells, ozonation would either not affect or would cause a slight increase in the relative ratio of GSH:GSSG. The second hypothesis also relies on the GSH:GSSG ratio, stating that because there is an excess of GSH, ozone exposure does not change the absolute amount of glutathione, just the percentage in the oxidized form. Our data suggest that rat type II pneumocytes respond using a combination of the two strategies. Exposure to hazardous levels of ozone causes both a small increase (+7%) in total cytoplasmic glutathione levels and a large increase (215%) in oxidized glutathione. The significance and scale of these changes are important to note. Studies in the literature often report only total glutathione levels (and use the unfortunate shorthand of calling it GSH rather than total glutathione), which masks potentially important data about the GSH:GSSG balance. Because the GSH/GSSG-Glo™ Assay detects total and oxidized glutathione, more information is gathered from each experiment and a more complete model developed.
In ozone exposure studies, experimental conditions are achieved by exposing cells to different gas environments in different physical locations. Because of this, the different gas treatments that comprise a single experiment cannot be performed in adjacent wells of a 96-well plate. It is critical, therefore, that the biochemical analyses performed are flexible and include internal controls so that data can be compared between cells treated at different times, as well as during different runs. The GSH/GSSG-Glo™ Assay provides a straight-forward approach to examining cellular redox state that works well with this type of experimental design.
Additional advantages of the GSH/GSSG-Glo™ Assay are that all steps occur in the cell culture wells, reducing stress that might occur during scraping or trypsinization to release cells and sample loss during manipulations. The luminescent signal is proportional to the amount of glutathione within the sample and stable for 3 hours, allowing flexibility in data collection schemes. Also, unlike other assays available, all of the reagents needed to measure GSSG and total glutathione are included and the assay determines GSSG levels directly in the well, without additional deproteination steps, extractions or HLPC separation.
The rapid and simple protocol allows increased flexibility in the biochemical analyses that can be performed during a single ozone exposure setup. In fact, the GSH/GSSG-Glo™ Assay can be performed using the same cell population in parallel wells during the 1.5-hour incubation period within the ApoTox-Glo™ Assay, an assay that assesses cell viability, cytotoxicity and apoptosis events in the same cell population by measuring a protease activity specific to live cells, a protease activity that is released from cells that have lost membrane integrity and caspase-3/7 activity. This approach provides additional time savings and confidence in the data trends across biochemical assays since the cells used were all plated, exposed and processed at the same time.
Conclusion
By exposing L2 cells to 350ppb O3 and characterizing changes using the GSH/GSSG-Glo™ Assay, we determined that type II alveolar cells' response to oxidative stress includes changes in the total amount and oxidative state of cytoplasmic glutathione. Our findings indicate that full characterization of alveolar cells’ redox homeostasis mechanisms will require attention to absolute and relative concentrations of the components involved. The GSH/GSSG-Glo™ Assay determines total and oxidized glutathione levels, is exceedingly easy to use and can be performed in cell-culture-treated 96-well plates, making it an ideal choice for experiments requiring technically difficult exposure parameters and where high-throughput or multiplexing would augment analysis.
Related Products
Related Protocols
Article References
- Chen, C. et al. (2007) Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ. Health Perspect. 115, 1732–7.
- TenHoor, T., Mannino, D.M. and Moss, M. (2001) Risk factors for ARDS in the United States: Analysis of the 1993 National Mortality Followback Study. Chest 119, 1179–84.
- Urata, Y. et al. (2006) 17Beta-estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J. Biol. Chem. 281, 13092–102.
- Rahman, I. and MacNee, W. (2000) Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 16, 534–54.
- Klaunig, J.E., Kamendulis, L.M. and Hocevar, B.A. (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 38, 96–109.
- Brink, C.B. et al. (2008) Studies on cellular resilience and adaptation following acute and repetitive exposure to ozone in cultured human epithelial (HeLa) cells. Redox Report 13, 87–100.
- Hollingsworth, J.W., Kleeberger, S.R. and Foster, W.M. (2007) Ozone and pulmonary innate immunity. Proc. Am. Thorac. Soc. 4, 240–6.
- Pryor, W.A. (1994) Mechanisms of radical formation from reactions of ozone with target molecules in the lung. Free Radic. Biol. Med. 17, 451–65.
- Nadadur, S.S. et al. (2005) Acute ozone-induced differential gene expression profiles in rat lung. Environ. Health Perspect. 113, 1717–22.
- Todokoro, M. et al. (2004) Effect of ozone exposure on intracellular glutathione redox state in cultured human airway epithelial cells. Inflammation 28, 105–14.
- Duan, X. et al. (1996) Ozone-induced alterations in glutathione in lung subcompartments of rats and monkeys. Am. J. Respir. Cell Mol. Biol. 14, 70–5.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Chalfant, M. and Bernd, K. Detecting Ozone-Induced Changes in Cellular Redox Balance via GSH/GSSG-Glo™ Assay. [Internet] 2011. [cited: year, month, date]. Available from: https://www.promega.com/es-es/resources/pubhub/tpub_059-detecting-ozone-induced-changes-in-cellular-redox-balance-via-gsh-gssg-glo-assay/
American Medical Association, Manual of Style, 10th edition, 2007
Chalfant, M. and Bernd, K. Detecting Ozone-Induced Changes in Cellular Redox Balance via GSH/GSSG-Glo™ Assay. Promega Corporation Web site. https://www.promega.com/es-es/resources/pubhub/tpub_059-detecting-ozone-induced-changes-in-cellular-redox-balance-via-gsh-gssg-glo-assay/ Updated 2011. Accessed Month Day, Year.
ApoTox-Glo and GSH/GSSG-Glo are trademarks of Promega Corporation.
FLx800 is a trademark of BioTek Instruments, Inc.